You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how many grams of c6h12o6 are needed to be dissolved on Google, you do not find the information you need! Here are the best content compiled and compiled by the https://chewathai27.com team, along with other related topics such as: how many grams of c6h12o6 are needed to be dissolved how many grams of c6h12o6 are needed to be dissolved in water to make 100 grams of a 250 ppm, how many grams of glucose (c6h12o6 are needed to prepare), how many grams of glucose are needed to prepare 400ml of 2 glucose solution, how many grams of glucose are needed to prepare 400ml of a 2.0 (m/v) glucose solution, how many grams of glucose (c6h12o6) are needed to make 2000 g of a 2.8 glucose m/m solution, how many grams of kno3 should be dissolved in water to make 500.0 grams of a 20.0 ppm solution?, how many grams of glucose (c6h12o6) are contained in 555 ml of a 1.77 m glucose solution, how many grams of glucose c6h12o6 brainly
Contents
How many particles will C6H12O6 break into?
The answer is A because C6H12O6 breaks into 1 particles, the least of any of the choices. The fewer particles that a solute ionizes into, the lower the boiling point will be.
What is the concentration of C6H12O6?
As we know the molar mass of glucose is 180g. Therefore, the molarity of the glucose solution is: 0.9/180 = 0.005 moles/L.
What occurs when glucose C6H12O6 dissolves in water?
Glucose dissolves in water because polar water molecules attach to the glucose molecules. When a glucose molecule (centre) is placed into water the negatively charged oxygen ions (red) attract and surround the positively charged O-H (hydroxyl- ) groups in glucose, forming hydrogen bonds (white).
Can glucose be dissolved in water?
What is C6H12O6?
D-Glucose | C6H12O6 – PubChem.
How many particles does C6H12O6?
Answer and Explanation: There are 24 atoms in one molecule of C6 H12 06. This chemical compound has 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen.
How many molecules of hydrogen are c6h12o6?
Molecules of glucose (blood sugar) contain 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
How many atoms of carbon are in a molecule of glucose c6h12o6?
This molecule of the sugar glucose consists of 6 carbon atoms bonded together as a chain with additional atoms of oxygen and hydrogen.
What c6h12o6 4?
Dextrose (D-Glucose) L-Glucose.
What happens to the concentration of C6H12O6?
Thus, the increased concentration of C6H12O6 C 6 H 12 O 6 gets balanced and the equilibrium constant remains fixed at a constant temperature. Hence, the equilibrium will shift left if the concentration of C6H12O6 C 6 H 12 O 6 is increased.
How do I find the molarity of a solution?
The most common way to express solution concentration is molarity (M), which is defined as the amount of solute in moles divided by the volume of solution in liters: M = moles of solute/liters of solution.
What happens if glucose and water are mixed?
Answer: As the sugar dissolves in water, the crystals break down, releasing tiny particles. Sugar particles pass through the holes between the water particles and combine to form a sugar syrup.
Is C6H12O6 a solid liquid or gas?
It can occur either in the solid or liquid form. It is water-soluble and is also soluble in acetic acid.
What happens when glucose reacts with water?
Due to the dipole moment of water, glucose form hydrogen bond with water and hydrogen enthalpy of glucose is sufficient to make it soluble in water [36] . The glucose molecule converts from solid to the aqueous form as: C 6 H 12 O 6 +H 2 O → C 6 H 12 O 6 (aq). …
Why is C6H12O6 not an electrolyte?
Glucose, a sugar with the chemical formula C6H12O6, is a typical example of a nonelectrolyte. Glucose (commonly known as sugar) dissolves readily in water, but because it does not dissociate inside the solution into ions, it is considered a nonelectrolyte.
Attention Required! | Cloudflare
- Article author: www.toppr.com
- Reviews from users: 44803
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about Attention Required! | Cloudflare Updating …
- Most searched keywords: Whether you are looking for Attention Required! | Cloudflare Updating
- Table of Contents:
Please complete the security check to access wwwtopprcom
Why do I have to complete a CAPTCHA
What can I do to prevent this in the future

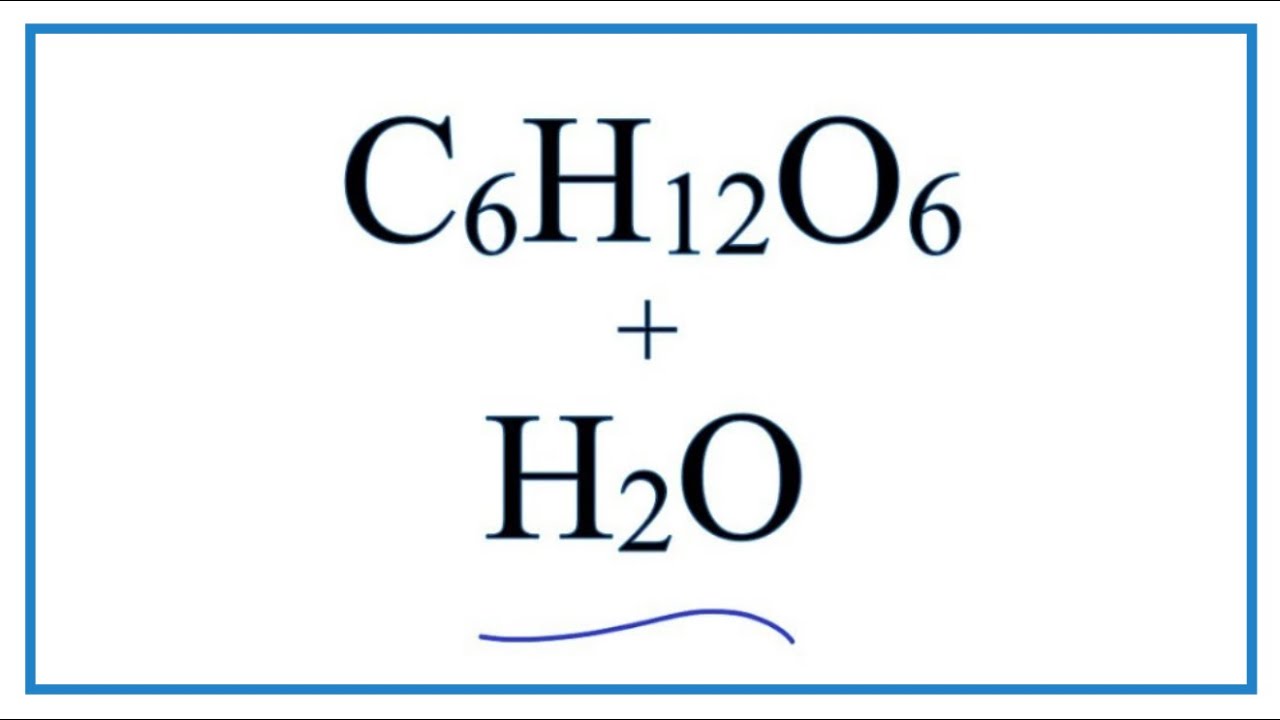
Equation for Glucose Dissolving in Water (C6H12O6 + Water) – YouTube
- Article author: www.youtube.com
- Reviews from users: 4082
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about Equation for Glucose Dissolving in Water (C6H12O6 + Water) – YouTube Updating …
- Most searched keywords: Whether you are looking for Equation for Glucose Dissolving in Water (C6H12O6 + Water) – YouTube Updating In this video we will describe the equation C6H12O6 + H2O and write what happens when C6H12O6 is dissolved in water.When C6H12O6 is dissolved in H2O (water) …breslyn, PlusH2O, C6H12O6 + Water, Glucose plus Water, equation for C6H12O6 + water, how to balance C6H12O6 + water, Glucosee plus water chemical equation, C6H12O6 and water chemistry, C6H12O6 + water product, C6H12O6 + H2O reaction, what does C6H12O6 + H2O make
- Table of Contents:
How to Convert Moles of C6H12O6 to Grams – YouTube
- Article author: www.youtube.com
- Reviews from users: 17692
Ratings
- Top rated: 4.8
- Lowest rated: 1
- Summary of article content: Articles about How to Convert Moles of C6H12O6 to Grams – YouTube Updating …
- Most searched keywords: Whether you are looking for How to Convert Moles of C6H12O6 to Grams – YouTube Updating In this video well learn to convert moles of C6H12O6 to grams. The technique used can be applied to any mole to gram conversion. For our practice problem we’…breslyn, Moles C6H12O6 to grams, grams C6H12O6to moles, C6H12O6 moles, Glucose moles to grams, convert moles C6H12O6 to grams, change moles of C6H12O6 to grams, C6H12O6ro moles C6H12O6, C6H12O6 is how many grams, C6H12O6 conversion, how many moles is Glucose
- Table of Contents:

how many grams of c6h12o6 are needed to be dissolved
- Article author: norcohs.cnusd.k12.ca.us
- Reviews from users: 22383
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about how many grams of c6h12o6 are needed to be dissolved Updating …
- Most searched keywords: Whether you are looking for how many grams of c6h12o6 are needed to be dissolved Updating
- Table of Contents:

brainly.in
- Article author: brainly.in
- Reviews from users: 29874
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about brainly.in Updating …
- Most searched keywords: Whether you are looking for brainly.in Updating
- Table of Contents:

brainly.com
- Article author: brainly.com
- Reviews from users: 4121
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about brainly.com Answer: 0.025 g C6H12O6. Explanation: ppm = (g solute/ g solution)* 10^6. g solute= (ppm * g solution)/ 10^6. g solute = (250 ppm * 100 g)/10^6. …
- Most searched keywords: Whether you are looking for brainly.com Answer: 0.025 g C6H12O6. Explanation: ppm = (g solute/ g solution)* 10^6. g solute= (ppm * g solution)/ 10^6. g solute = (250 ppm * 100 g)/10^6.
- Table of Contents:

Error 403 (Forbidden)
- Article author: www.quora.com
- Reviews from users: 2326
Ratings
- Top rated: 3.5
- Lowest rated: 1
- Summary of article content: Articles about Error 403 (Forbidden) If you want to prepare 400mL of solution – you require 5g * 4 = 20g glucose dissolved in water to a final volume of 400mL. …
- Most searched keywords: Whether you are looking for Error 403 (Forbidden) If you want to prepare 400mL of solution – you require 5g * 4 = 20g glucose dissolved in water to a final volume of 400mL.
- Table of Contents:

How many grams of C6H12O6 need to be dissolved in 350g of water vto have a solution which
- Article author: www.jiskha.com
- Reviews from users: 31272
Ratings
- Top rated: 4.4
- Lowest rated: 1
- Summary of article content: Articles about How many grams of C6H12O6 need to be dissolved in 350g of water vto have a solution which No, I obtained an answer of 84.4 g C6H12O6. molality = mols/kg. 1.34 molal = mols/0.350 kg 1.34*0.350 = mols = 0.469 mols = grams/molar mass …
- Most searched keywords: Whether you are looking for How many grams of C6H12O6 need to be dissolved in 350g of water vto have a solution which No, I obtained an answer of 84.4 g C6H12O6. molality = mols/kg. 1.34 molal = mols/0.350 kg 1.34*0.350 = mols = 0.469 mols = grams/molar mass
- Table of Contents:
Respond to this Question
Similar Questions
Still need help

How many grams of glucose C6H12O6 should be dissolved class 11 chemistry CBSE
- Article author: www.vedantu.com
- Reviews from users: 2663
Ratings
- Top rated: 3.3
- Lowest rated: 1
- Summary of article content: Articles about How many grams of glucose C6H12O6 should be dissolved class 11 chemistry CBSE How many grams of glucose C6H12O6 should be dissolved in 0.5 kg of water at 25 oC to reduce the vapor pressure of the water by 1.0 % ? A. 50.5 g. B. 50.0 g. C. …
- Most searched keywords: Whether you are looking for How many grams of glucose C6H12O6 should be dissolved class 11 chemistry CBSE How many grams of glucose C6H12O6 should be dissolved in 0.5 kg of water at 25 oC to reduce the vapor pressure of the water by 1.0 % ? A. 50.5 g. B. 50.0 g. C. How many grams of glucose C6H12O6 should be dissolved in 05 kg of water at 25 oC to reduce the vapor pressure of the water by 10 A 505 g B 500 g C 180 g D 182 g
- Table of Contents:

Answered: How many grams of glucose (C6H12O6)… | bartleby
- Article author: www.bartleby.com
- Reviews from users: 20128
Ratings
- Top rated: 3.7
- Lowest rated: 1
- Summary of article content: Articles about Answered: How many grams of glucose (C6H12O6)… | bartleby Solution for How many grams of glucose (C6H12O6) would you need to prepare 2.0 l of 2% glucose solution? …
- Most searched keywords: Whether you are looking for Answered: How many grams of glucose (C6H12O6)… | bartleby Solution for How many grams of glucose (C6H12O6) would you need to prepare 2.0 l of 2% glucose solution? Solution for How many grams of glucose (C6H12O6) would you need to prepare 2.0 l of 2% glucose solution?
- Table of Contents:

See more articles in the same category here: https://chewathai27.com/toplist.
How many grams of C6H12O6 need to be dissolved in 350g of water vto have a solution which
How many grams of C6H12O6 need to be dissolved in 350g of water vto have a solution which freezes at -2.50 degrees celcius?
delta T = K f *m
You know K f , m = molality = mols/kg and mols = grams/molar mass, delta T is 2.50. Solve for grams. Post your work if you get stuck.
what is delta t i never learned that
Delta T is T normal f.p. – T new f.p. . Delta T in this problem is 2.50 degrees C or since the normal freezing point is 0 and you want it to be -2.50, that is 0 – (-2.50) = 2.50.
i really don’t understand can you help me throught the first part… what do you make moles? i really need help i have a big test tommorrow!!
delta T = i*K f *m
delta T = 2.50
i = 1
K f = 1.86
solve for m
2.50 = 1*1.86*m
m = 2.50/(1*1.86) = 1.34 molal.
The definition of molality = #mols/kg.
m=mols/kg
1.34 molal = mols/0.350 kg
1.34*0.350 = mols = 0.469
mols = grams/molar mass
0.469 = g/180
g = 0.469*180 = 84.4 g C6H12O6.
Let me know if this isn’t clear. Check my thinking. Check my arithmetic.
okay so do i do the exact same thing for the other one but i=3
For the CaCl2 problem, i = 3, Kf is the same, I think kg solvent is not 0.350 kg but something else. Otherwise, yes, the same procedure.
did you get 13.92 g
No, I obtained an answer of 84.4 g C6H12O6.
molality = mols/kg.
1.34 molal = mols/0.350 kg
1.34*0.350 = mols = 0.469
mols = grams/molar mass
0.469 = g/180
g = 0.469*180 = 84.4 g C6H12O6.
no for the other one sorry!! 13.92!!
You’re mixing yourself up AND me by not posting the answers with the question. Let’s keep it simple. Answer the question with THAT post and not some other post. I didn’t get 13.92.
If you will show your work I can find your error.
2.0=1.86(3)M
TIMES 1.86 TIMES 3=5.58 THEN DIVIDE
2.0/5.58 =.358
.358 TIMES .350 =.1254
THEN .1254 TIMES 110.98 (THAT IS THE MOLAR MASS OF CACL2) = 13.92
If you will go back and read the original problem, I believe it is in 100 g water, not 350 g water.
So 0.358*0.100*110.98 = ?? and I have 3.97 g CaCl2.
thank you so much!!
How many grams of glucose C6H12O6 should be dissolved class 11 chemistry CBSE
Hint: There is a formula to calculate the mole of glucose which is present in the 0.5 kg of water and it is as follows.
\[\text{mole fraction of the solute =}\dfrac{n}{n+N}\]
Here n = number of moles of the solute
N = number of moles of the water.
Complete Solution :
– In the question it is given that to calculate the number of grams of glucose is required to reduce the vapor pressure of the 0.5 kg of water by 1%.
– Mass of the glucose = 180.
– Assume the moles of glucose are ‘n’.
– Then number of moles water = $\dfrac{500g}{18}=27.77 moles$
– The number of moles of water in 0.5 kg of water N = 27.77 moles.
– The vapor pressure should be reduced by 1% means $\dfrac{0.01{{P}^{o}}}{{{P}^{o}}}$ .
– Here ${{P}^{o}}$ = Vapor pressure of the water.
– Therefore vapor pressure of the water is equal to number of moles of glucose, then
\[\dfrac{n}{n+N}=\dfrac{0.01{{P}^{o}}}{{{P}^{o}}}\]
– Now substitute N value in the above formula to get the number of moles of glucose.
\[\begin{align}
& \dfrac{n}{n + N}=\dfrac{0.01{{P}^{o}}}{{{P}^{o}}} \\
& \dfrac{n}{n + 27.7}=\dfrac{1}{100} \\
& n=0.28moles \\
\end{align}\]
– The number of moles of glucose required to decrease the vapor pressure of the 0.5 kg of water is 0.28 moles.
– But we need the mass of the glucose required to reduce the vapor pressure of the 0.5 kg of water is 0.28 moles.
– Therefore the mass of the glucose required is = (0.28) (180) = 50.5 g.
So, the correct answer is “Option B”.
Note: Whenever we are going to add a non-volatile solute to water, the vapor pressure of the water is going to decrease. Because the non-volatile solute occupies the surface of the water and won’t allow the water to evaporate.
So you have finished reading the how many grams of c6h12o6 are needed to be dissolved topic article, if you find this article useful, please share it. Thank you very much. See more: how many grams of c6h12o6 are needed to be dissolved in water to make 100 grams of a 250 ppm, how many grams of glucose (c6h12o6 are needed to prepare), how many grams of glucose are needed to prepare 400ml of 2 glucose solution, how many grams of glucose are needed to prepare 400ml of a 2.0 (m/v) glucose solution, how many grams of glucose (c6h12o6) are needed to make 2000 g of a 2.8 glucose m/m solution, how many grams of kno3 should be dissolved in water to make 500.0 grams of a 20.0 ppm solution?, how many grams of glucose (c6h12o6) are contained in 555 ml of a 1.77 m glucose solution, how many grams of glucose c6h12o6 brainly

