You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how many grams of nh3 can be produced on Google, you do not find the information you need! Here are the best content compiled and compiled by the https://chewathai27.com team, along with other related topics such as: how many grams of nh3 can be produced how many grams of nh3 can be produced from 12.0 g of h2, how many grams of nh3 can be produced from 4.09 mol of n2 and excess h2, if you have 3.64 g of h2, how many grams of nh3 can be produced, how many grams of nh3 can be prepared from 77.3 grams of n2, how many grams of nh3 will be produced if 122 g n2 reacts completely with h2?, how many grams of ammonia nh3 are produced in the reaction with 50.0 g of n2 nitrogen, how many grams of nh3 can be produced from the reaction of 28 g of n2 and 25 g of h2, how many grams of nh3 can be produced from 3.48 mol of n2 and excess h2
Contents
How many grams of NH3 can be made according to the reaction?
Answer: 34 g of NH3 can be produced.…
How many grams is NH3?
17.03052 g/mol .
How many moles of NH3 are produced?
The moles of NH3 produced are 14 moles.
What is the mass of NH3 produced?
Ammonia’s molecular formula is NH3. Its molar mass is 17.0306g.
How many grams of N2 are needed to produce 2.17 mol of NH3 when reacted according to this equation?
How many grams of N 2 are needed to produce 2.17 mol of NH 3 when reacted according to this chemical equation? 30.4 g (Note: here we go from a product to a reactant, showing that mole-mass problems can begin and end with any substance in the chemical equation.)
How much ammonia can produce from the given amount of nitrogen gas?
The balanced equation shows that one mole of nitrogen (N2) will produce two moles of ammonia.
What mass in grams of NH3 can be produced from 50.0 g of n2?
We have 50 g of nitrogen gas here, so we need to find the number of moles of nitrogen. From here, we would produce 1.79⋅2=3.58 moles of ammonia. 3.58mol ⋅17.031 gmol ≈61 g of NH3 .
How many molecules does NH3?
The molar mass of NH3 is equalled to 17 gram. 0.5 moles of NH3 are present. 0.5×6.022×10^23 = 3.011×10^23 molecules of NH3. Each NH3 molecule has 4 atoms.
How many moles of NH3 are produced if 2.5 grams of N2 reacts?
We end up with 5 moles of NH3.
How many moles of NH3 can be produced from 12 moles H2 and N2?
Therefore, 12 moles of hydrogen makes 8 moles of ammonia.
How many grams of ammonia are produced from 3.0 moles of nitrogen?
1 Answer. Nam D. Nitrogen is the limiting reactant, and 3.74 grams of ammonia is formed.
How is NH3 formed?
Ammonia is formed from 3 atoms of Hydrogen and 1 atom of Nitrogen. Hence, three hydrogen atoms each share their 1 electron with nitrogen to form three covalent bonds and make an ammonia molecule (NH3) ammonia molecule.
How many grams of NH3 are produced from the reaction of 56 grams nitrogen gas and an abundance of hydrogen gas?
56g N2 * (1 mol N2/ 28g N2) * (2 mol NH3/1 mol N2) * (17g NH3/1 mol NH3) = 68g NH3 produced.
How is NH3 made?
Ammonia is produced commercially via the catalytic reaction of nitrogen and hydrogen at high temperature and pressure.
How many grams of ammonia NH3 are produced in the reaction with 50.0 g of N2 nitrogen?
We have 50 g of nitrogen gas here, so we need to find the number of moles of nitrogen. From here, we would produce 1.79⋅2=3.58 moles of ammonia. 3.58mol ⋅17.031 gmol ≈61 g of NH3 .
How many grams of P2O3 are required to produce 10.2 moles H3PO3?
561 g P2O3 are required to produce 10.2 mol H3PO3 .
How many grams of hydrogen are necessary to react completely with 50.0 g of nitrogen?
Therefore, 10.7 grams of Hydrogen are necessary to fully react with 50.0 grams of Nitrogen.
What is the ratio of moles of H2 G consumed to moles of NH3 G produced?
So, the ratio of hydrogen to ammonia is 3:2.
Error 403 (Forbidden)
- Article author: www.quora.com
- Reviews from users: 29472
Ratings
- Top rated: 4.4
- Lowest rated: 1
- Summary of article content: Articles about Error 403 (Forbidden) The theoretical yield is 34 gram and that is the maximum possible. Typically you will get about 10 gram before the reaction mixture reaches equilibrium even … …
- Most searched keywords: Whether you are looking for Error 403 (Forbidden) The theoretical yield is 34 gram and that is the maximum possible. Typically you will get about 10 gram before the reaction mixture reaches equilibrium even …
- Table of Contents:

Error 403 (Forbidden)
- Article author: www.quora.com
- Reviews from users: 21002
Ratings
- Top rated: 3.2
- Lowest rated: 1
- Summary of article content: Articles about Error 403 (Forbidden) Updating …
- Most searched keywords: Whether you are looking for Error 403 (Forbidden) Updating
- Table of Contents:

What is the molar mass of ammonia, “NH”_3? | Socratic
- Article author: socratic.org
- Reviews from users: 25766
Ratings
- Top rated: 3.2
- Lowest rated: 1
- Summary of article content: Articles about What is the molar mass of ammonia, “NH”_3? | Socratic Updating …
- Most searched keywords: Whether you are looking for What is the molar mass of ammonia, “NH”_3? | Socratic Updating “17.03052 g/mol”. To determine the molar mass of any compound, all you have to do is add up the molar masses of every atom that makes up the respective compound. In this case, you know that ammonia, “NH”_3, is composed of one nitrogen atom, “N” three hydrogen atoms, “H” This means that its molar mass will be the sum of the molar mass of one nitrogen atom and three times the molar mass of a hydrogen atom. A quick look in the periodic table will show that you have “N: ” “14.0067 g/mol” “H: ” “1.00794 g/mol” The molar mass of ammonia will thus be “14.0067 g/mol” + 3 xx “1.00794 g/mol” = color(green)(“17.03052 g/mol”)
- Table of Contents:

How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2? – Home Work Help – Learn CBSE Forum
- Article author: ask.learncbse.in
- Reviews from users: 45628
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2? – Home Work Help – Learn CBSE Forum Updating …
- Most searched keywords: Whether you are looking for How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2? – Home Work Help – Learn CBSE Forum Updating Part A: How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2?
Part B: How many grams of NH3 can be produced from 3.60 mol of N2 and excess H2?
Part C: How many grams of H2 are needed to produce 11.76… - Table of Contents:
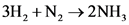
how many grams of nh3 can be produced
- Article author: www.calstatela.edu
- Reviews from users: 35712
Ratings
- Top rated: 3.7
- Lowest rated: 1
- Summary of article content: Articles about how many grams of nh3 can be produced Updating …
- Most searched keywords: Whether you are looking for how many grams of nh3 can be produced Updating
- Table of Contents:

N2(g) + 3H2-> 2NH3(g) How many grams of ammonia (NH3) can be produced from the reaction of 28.0 g of N2 and 25.0 g of H2? | Wyzant Ask An Expert
- Article author: www.wyzant.com
- Reviews from users: 7971
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about N2(g) + 3H2-> 2NH3(g) How many grams of ammonia (NH3) can be produced from the reaction of 28.0 g of N2 and 25.0 g of H2? | Wyzant Ask An Expert N2(g) + 3H2-> 2NH3(g) How many grams of ammonia (NH3) can be produced from the reaction of 28.0 g of N2 and 25.0 g of H2? …
- Most searched keywords: Whether you are looking for N2(g) + 3H2-> 2NH3(g) How many grams of ammonia (NH3) can be produced from the reaction of 28.0 g of N2 and 25.0 g of H2? | Wyzant Ask An Expert N2(g) + 3H2-> 2NH3(g) How many grams of ammonia (NH3) can be produced from the reaction of 28.0 g of N2 and 25.0 g of H2? N2(g) + 3H2-> 2NH3(g) How many grams of ammonia (NH3) can be produced from the reaction of 28.0 g of N2 and 25.0 g of H2?
- Table of Contents:
1 Expert Answer
Still looking for help Get the right answer fast
 Read More
Read More
How many grams of NH3 can be produced from 2.44 mol of N2 and excess H2? | Socratic
- Article author: socratic.org
- Reviews from users: 4708
Ratings
- Top rated: 3.6
- Lowest rated: 1
- Summary of article content: Articles about How many grams of NH3 can be produced from 2.44 mol of N2 and excess H2? | Socratic Warning! Long Answer. (a) 83.1 g; (b) 2.264 g; (c) 3.17×1019lmolecules. Explanation: (a) Mass of ammonia. You know that you will need a … …
- Most searched keywords: Whether you are looking for How many grams of NH3 can be produced from 2.44 mol of N2 and excess H2? | Socratic Warning! Long Answer. (a) 83.1 g; (b) 2.264 g; (c) 3.17×1019lmolecules. Explanation: (a) Mass of ammonia. You know that you will need a … Warning! Long Answer. (a) 83.1 g; (b) 2.264 g; (c) 3.17 × 10^19color(white)(l)”molecules” > (a) Mass of ammonia You know that you will need a balanced chemical equation with masses, moles, and molar masses, etc. (i). Assemble all the information in one place. M_text(r):color(white)(mm)28.01color(white)(m)2.016color(white)(mll) 17.03 color(white)(mmmmm)”N”_2+ color(white)(l)”3H”_2 → “2NH”_3 n”/mol”: color(white)(m)2.44 (ii). Calculate the moles of “NH”_3 “Moles of NH”_3 = 2.44 color(red)(cancel(color(black)(“mol N”_2))) × (“2 mol NH”_3)/(1 color(red)(cancel(color(black)(“mol N”_2)))) = 4.88 color(white)(l) “mol NH”_3 (iii). Calculate the mass of “NH”_3 “Mass of NH”_3 = 4.88 color(red)(cancel(color(black)(“mol NH”_3)))× (“17.03 g NH”_3)/(1 color(red)(cancel(color(black)(“mol NH”_3)))) = “83.1 g NH”_3 (b) Mass of “H”_2 (i). Calculate the moles of “NH”_3 “Moles of NH”_3 = 12.75 color(red)(cancel(color(black)(“g NH”_3))) × “1 mol NH”_3/(17.03 color(red)(cancel(color(black)(“g NH”_3)))) = “0.748 68 mol NH”_3 (ii). Calculate the moles of “H”_2 “Moles of H”_2 = “0.748 68” color(red)(cancel(color(black)(“mol NH”_3))) × “3 mol H”_2/(2 color(red)(cancel(color(black)(“mol NH”_3)))) = “1.1230 mol H”_2 (iii). Calculate the mass of “H”_2 “Mass of H”_2 = 1.1230 color(red)(cancel(color(black)(“mol H”_2))) × “2.016 g H”_2/(1 color(red)(cancel(color(black)(“mol H”_2)))) = “2.264 g H”_2 (c). Molecules of “NH”_3 (i). Calculate the moles of “H”_2 “Moles of H”_2 = 1.59 × 10^”-4″ color(red)(cancel(color(black)(“g H”_2))) × “1 mol H”_2/(2.016 color(red)(cancel(color(black)(“g H”_2)))) = 7.887 × 10^”-5″color(white)(l) “mol H”_2 (ii). Calculate the moles of “NH”_3 ” Moles of NH”_3 = 7.887 × 10^”-5″ color(red)(cancel(color(black)(“mol H”_2))) × “2 mol NH”_3/(3 color(red)(cancel(color(black)(“mol H”_2)))) = 5.258 ×10^”-5″color(white)(l) “mol NH”_3 (iii). Calculate the molecules of “NH”_3 “Molecules of NH”_3 = 5.258 ×10^”-5″ color(red)(cancel(color(black)(“mol NH”_3))) × (6.022 × 10^23 color(white)(l)”molecules NH”_3)/( 1 color(red)(cancel(color(black)(“mol NH”_3)))) = 3.17 × 10^19color(white)(l)”molecules NH”_3#
- Table of Contents:

“How many grams of NH3 can be produced from the reaction of 28 g of N2 and 25 g of H2? N2
- Article author: www.jiskha.com
- Reviews from users: 3773
Ratings
- Top rated: 3.9
- Lowest rated: 1
- Summary of article content: Articles about “How many grams of NH3 can be produced from the reaction of 28 g of N2 and 25 g of H2? N2 grams NH3 = 2.0 x 17 = 34 g. moles H2 = 25/2 = 12.5 moles. moles NH3 produced = 12.5 x (2 moles NH3/3 moles H2) = 8.33 … …
- Most searched keywords: Whether you are looking for “How many grams of NH3 can be produced from the reaction of 28 g of N2 and 25 g of H2? N2 grams NH3 = 2.0 x 17 = 34 g. moles H2 = 25/2 = 12.5 moles. moles NH3 produced = 12.5 x (2 moles NH3/3 moles H2) = 8.33 …
- Table of Contents:
Respond to this Question
Similar Questions
Still need help

brainly.com
- Article author: brainly.com
- Reviews from users: 6791
Ratings
- Top rated: 3.0
- Lowest rated: 1
- Summary of article content: Articles about brainly.com Grams of NH3 = moles of NH3 * molar mass of NH3 = 8.16 mole * 17 g/mole = 139 g. 139 grams of nh3 can be produced from 4.08 mol of n2 and excess h2. …
- Most searched keywords: Whether you are looking for brainly.com Grams of NH3 = moles of NH3 * molar mass of NH3 = 8.16 mole * 17 g/mole = 139 g. 139 grams of nh3 can be produced from 4.08 mol of n2 and excess h2.
- Table of Contents:

brainly.in
- Article author: brainly.in
- Reviews from users: 30549
Ratings
- Top rated: 3.3
- Lowest rated: 1
- Summary of article content: Articles about brainly.in How many grams of NH3 can be prepared from 77.3 grams of N2 and 14.2 grams of H2?(Hint: N, +3H, → 2NH3) 80.4 g 79.7 g 47.0 g 120.0 g · Thus, … …
- Most searched keywords: Whether you are looking for brainly.in How many grams of NH3 can be prepared from 77.3 grams of N2 and 14.2 grams of H2?(Hint: N, +3H, → 2NH3) 80.4 g 79.7 g 47.0 g 120.0 g · Thus, …
- Table of Contents:

Access to this page has been denied.
- Article author: www.chegg.com
- Reviews from users: 22860
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about Access to this page has been denied. 1. How many grams of NH3 can be produced from 3.18 mol of N2 and excess H2? 2. How many grams of H2 are needed to produce 10.05 g of NH3? 3. How … …
- Most searched keywords: Whether you are looking for Access to this page has been denied. 1. How many grams of NH3 can be produced from 3.18 mol of N2 and excess H2? 2. How many grams of H2 are needed to produce 10.05 g of NH3? 3. How …
- Table of Contents:

how many gram of nh3 will be produce when 50g n2 react with h2 – Chemistry – TopperLearning.com | t0qegjww
- Article author: www.topperlearning.com
- Reviews from users: 45300
Ratings
- Top rated: 4.9
- Lowest rated: 1
- Summary of article content: Articles about how many gram of nh3 will be produce when 50g n2 react with h2 – Chemistry – TopperLearning.com | t0qegjww how many gram of nh3 will be produce when 50g n2 react with h2 – Chemistry – TopperLearning.com | t0qegjww. …
- Most searched keywords: Whether you are looking for how many gram of nh3 will be produce when 50g n2 react with h2 – Chemistry – TopperLearning.com | t0qegjww how many gram of nh3 will be produce when 50g n2 react with h2 – Chemistry – TopperLearning.com | t0qegjww. CBSE Class , Class CBSE, CBSE th class, th class CBSE, CBSE th, TopperLearninghow many gram of nh3 will be produce when 50g n2 react with h2 – Chemistry – TopperLearning.com | t0qegjww
- Table of Contents:

How many grams of NH3 can be produced from 4.84 mol of N2? Access 14 best answers & solutions.
- Article author: www.accessify.com
- Reviews from users: 37842
Ratings
- Top rated: 3.6
- Lowest rated: 1
- Summary of article content: Articles about How many grams of NH3 can be produced from 4.84 mol of N2? Access 14 best answers & solutions. Here are 14 best answers to ‘How many grams of NH3 can be produced from 4.84 mol … N2 + 2H2 –> 2NH3 As you can see that the mole ratio is N2 : NH3 1 : 2 … …
- Most searched keywords: Whether you are looking for How many grams of NH3 can be produced from 4.84 mol of N2? Access 14 best answers & solutions. Here are 14 best answers to ‘How many grams of NH3 can be produced from 4.84 mol … N2 + 2H2 –> 2NH3 As you can see that the mole ratio is N2 : NH3 1 : 2 …
- Table of Contents:
Related Questions & Answers
Best solution
Other solutions
Answer in Inorganic Chemistry for aleeyah #127447
- Article author: www.assignmentexpert.com
- Reviews from users: 387
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about Answer in Inorganic Chemistry for aleeyah #127447 How many grams of NH3 can be produced from 2.49 mol of N2 and excess H2. Express your answer numerically in grams. … How many grams of H2 are … …
- Most searched keywords: Whether you are looking for Answer in Inorganic Chemistry for aleeyah #127447 How many grams of NH3 can be produced from 2.49 mol of N2 and excess H2. Express your answer numerically in grams. … How many grams of H2 are … free questions and answers, free answers, free solutions, get answers to questions, Chemistry questions, Chemistry answers, Inorganic Chemistry questions, Inorganic Chemistry answers
- Table of Contents:

See more articles in the same category here: https://chewathai27.com/toplist.
What is the molar mass of ammonia, #”NH”_3#?
To determine the molar mass of any compound, all you have to do is add up the molar masses of every atom that makes up the respective compound.
In this case, you know that ammonia, #”NH”_3# , is composed of
one nitrogen atom, #”N”#
nitrogen atom, three hydrogen atoms, #”H”#
This means that its molar mass will be the sum of the molar mass of one nitrogen atom and three times the molar mass of a hydrogen atom.
A quick look in the periodic table will show that you have
#”N: ” “14.0067 g/mol”#
#”H: ” “1.00794 g/mol”#
The molar mass of ammonia will thus be
How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2?
Part A: How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2?
Part B: How many grams of NH3 can be produced from 3.60 mol of N2 and excess H2?
Part C: How many grams of H2 are needed to produce 11.76g of NH3?
Part D: How many molecules (not moles) of NH3 are produced from 8.86 * 10^-4g of H2?
Concepts and reason
The question is based on the concept of limiting reagent.
Limiting reagent is a reagent in chemical reaction that gets totally consumed when chemical reaction is complete.
Fundamentals
There are two methods to determine the limiting reagent. One method is to find the mole ratio and then compare the mole ratio of reactants that get used in the reaction. Other method is to calculate the grams of products that are produced from the given quantities of reactants.
The mass of a substance can be calculated by using number of moles and molar mass of that substance as follows.
m = nM
Here, n is number of moles and M is molar mass of the substance.
Answer
Part A
2.10 moles of H2reacted with excess of N2. So H2 is a limiting reagent. The balanced chemical reaction can be written as follows:
The balanced chemical equation shows that 3 moles of H2 gives 2 moles ofNH3.
Part A
The moles of NH3 produced are 14 moles.
First write the balanced chemical equation. Then, solve the problem using unitary method.
Part B
2360 moles of N2reacted with excess of H2. So N2 is a limiting reagent. The balanced chemical reaction can be written as follows:
Now, mass of ammonia is calculated by using formula as follow.
Part B
The mass of NH3 produced is 122.6 g.
Part C
Write the balanced chemical reaction as follows:
Part C
The mass of H2 needed to produce 11.76 g of NH3 is 2.10 g.
First write the balanced chemical equation. Then, solve the problem using unitary method. After finding the moles of ammonia produced next step is to find the moles of hydrogen produced and with the help of this mass of hydrogen will be calculated.
Part D
Write the balanced chemical reaction as follows:
Part D
Molecules of NH3 produced are
First write the balanced chemical equation. Then, solve the problem using unitary method. After finding the moles of hydrogen produced next step is to find the molecules of ammonia produced.
N2(g) + 3H2-> 2NH3(g) How many grams of ammonia (NH3) can be produced from the reaction of 28.0 g of N2 and 25.0 g of H2?
N2(g) + 3H2-> 2NH3(g) This is the balanced equation
Note the mole ratio between N2, H2 and NH3. It is 1 : 3 : 2 This will be important.
moles N2 present = 28.0 g N2 x 1 mole N2/28 g = 1 mole N2 present
moles H2 present = 25.0 g H2 x 1 mole H2/2 g = 12.5 moles H2 present
Based on mole ratio, N2 is limiting in this situation because there is more than enough H2 but not enough N2.
moles NH3 that can be produced = 1 mole N2 x 2 moles NH3/mole N2 = 2 moles NH3 can be produced
grams of NH3 that can be produced = 2 moles NH3 x 17 g/mole = 34 grams of NH3 can be produced
NOTE: The key to this problem is recognizing that N2 is limiting, and therefore limits how much NH3 can be produced.
So you have finished reading the how many grams of nh3 can be produced topic article, if you find this article useful, please share it. Thank you very much. See more: how many grams of nh3 can be produced from 12.0 g of h2, how many grams of nh3 can be produced from 4.09 mol of n2 and excess h2, if you have 3.64 g of h2, how many grams of nh3 can be produced, how many grams of nh3 can be prepared from 77.3 grams of n2, how many grams of nh3 will be produced if 122 g n2 reacts completely with h2?, how many grams of ammonia nh3 are produced in the reaction with 50.0 g of n2 nitrogen, how many grams of nh3 can be produced from the reaction of 28 g of n2 and 25 g of h2, how many grams of nh3 can be produced from 3.48 mol of n2 and excess h2

