You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how many valence electrons are in the t-butyl ion ch33c+ on Google, you do not find the information you need! Here are the best content compiled and compiled by the Chewathai27.com team, along with other related topics such as: how many valence electrons are in the t-butyl ion ch33c+ how many valence electrons are in the t-butanol molecule ch33coh?, how many valence electrons are in the methane molecule ch4, how many valence electrons are in the peroxide ion, how many valence electrons are in the ethanol molecule ch3ch2oh, how many valence electrons are in the nitric acid molecule, how many valence electrons are in the sulfuric acid molecule h2so4
Contents
How many valence electrons are in the T butyl ion?
Since this molecule consists of 12 bones and each one contributes 22 electrons, two valence electrons. So we can say that this molecule consists to help multiply by two. That means 24 valence electrons.
How many valence electrons are in the T butanol molecule ch3 3coh?
So this gives us a total of 32 valence electrons.
How many valence electrons does Fhave?
For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge.
What is n butyl?
n-Butyl Functional Group
It consists of all four carbon atoms forming a chain and the rest of the molecule attaches at the first carbon. The n- stands for ‘normal’. In common names, the molecule would have n-butyl added to the molecule name. In systematic names, n-butyl would have butyl added to the molecule name.
What does butyl mean in organic chemistry?
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C 4H 9, derived from either of the two isomers (n-butane and isobutane) of butane.
What is a tert-butyl group?
Tert-butyl (tert-butyl group; tBu): A portion of a molecular structure which is equivalent to 2-methylpropane minus one hydrogen atom from carbon 2.
What is the triple point of t-butyl alcohol?
This error could have been avoided by observing the t-butyl alcohol carefully until it became solid to record a more accurate result. The triple point was 19 °C.
What is the IUPAC name of CH3 3COH?
tert-Butanol | (CH3)3COH – PubChem.
How do you find valence electrons?
Now that you know your element’s electron shells, finding the valence electrons is easy: just use the number of electrons in the outermost shell. If the outer shell is full (in other words, if it has eight electrons or, for the first shell, two), the element is inert and will not react easily with other elements.
How many valence electrons are in the fluoride ion?
That is, in this case, the valency of fluorine ions is -1. Since the last shell of fluoride ion has eight electrons, the valence electrons of fluoride ion are eight.
How many valence electrons are in the peroxide ion?
Transcript: This is the O2 2- Lewis structure. For the peroxide ion, Oxygen has six valence electrons. We have two Oxygens, and then we need to take in account these extra two valence electrons up here, so we’ll just add them for a total of 14 valence electrons for the 02 2- Lewis structure.
How many valence electrons are in the methane molecule ch4?
Example #1 – Methane CH. 4
This compound is covalent. This means there are 8 valence electrons, making 4 pairs, available.
Access to this page has been denied.
- Article author: www.chegg.com
- Reviews from users: 41831
Ratings
- Top rated: 3.0
- Lowest rated: 1
- Summary of article content: Articles about Access to this page has been denied. This problem has been solved! See the answerSee the answer See the answer done loading. How many valence electrons are in the t-butyl ion (CH) C. …
- Most searched keywords: Whether you are looking for Access to this page has been denied. This problem has been solved! See the answerSee the answer See the answer done loading. How many valence electrons are in the t-butyl ion (CH) C.
- Table of Contents:

SOLVED:How many valence electrons are in the t-butyl ion (CH;) C
- Article author: www.numerade.com
- Reviews from users: 26888
Ratings
- Top rated: 3.1
- Lowest rated: 1
- Summary of article content: Articles about SOLVED:How many valence electrons are in the t-butyl ion (CH;) C We have 123456789 10 11 12. Since this molecule consists of 12 bones and each one contributes 22 electrons, two valence electrons. So we can say … …
- Most searched keywords: Whether you are looking for SOLVED:How many valence electrons are in the t-butyl ion (CH;) C We have 123456789 10 11 12. Since this molecule consists of 12 bones and each one contributes 22 electrons, two valence electrons. So we can say … VIDEO ANSWER:in this question we have to tell number of valence electron in third beautiful iron. So inter tribute allow iron. We have to tell that how many valence electrons are present to attribute a lion. That is C. S. 33 C. Positive. So in this molecule we have to tell the number of valence electron present. First of all, let’s draw its structure. It has a structure of CH three. See then against CH. Three. It consists three metal group CH three CH three fine and positive charge on the central carbon. Now let’s calculate the number of bone present in this molecule. We have 123456789 10 11 12. Since this molecule consists of 12 bones and each one contributes 22 electrons, two valence electrons. So we can say that this molecule consists to help multiply by two. That means 24 valence electrons. So there are 24 valence electron in tertiary beautiful iron.
- Table of Contents:
Want better grades but can’t afford to pay for Numerade
Ask your parent or guardian for help
Share Question
Add To Playlist
92% of users report better grades
Log in to watch this video and 2000000 more!

SOLVED:How many valence electrons are in the t-butyl ion (CH;) C
- Article author: www.numerade.com
- Reviews from users: 41693
Ratings
- Top rated: 4.7
- Lowest rated: 1
- Summary of article content: Articles about SOLVED:How many valence electrons are in the t-butyl ion (CH;) C Updating …
- Most searched keywords: Whether you are looking for SOLVED:How many valence electrons are in the t-butyl ion (CH;) C Updating VIDEO ANSWER:in this question we have to tell number of valence electron in third beautiful iron. So inter tribute allow iron. We have to tell that how many valence electrons are present to attribute a lion. That is C. S. 33 C. Positive. So in this molecule we have to tell the number of valence electron present. First of all, let’s draw its structure. It has a structure of CH three. See then against CH. Three. It consists three metal group CH three CH three fine and positive charge on the central carbon. Now let’s calculate the number of bone present in this molecule. We have 123456789 10 11 12. Since this molecule consists of 12 bones and each one contributes 22 electrons, two valence electrons. So we can say that this molecule consists to help multiply by two. That means 24 valence electrons. So there are 24 valence electron in tertiary beautiful iron.
- Table of Contents:
Want better grades but can’t afford to pay for Numerade
Ask your parent or guardian for help
Share Question
Add To Playlist
92% of users report better grades
Log in to watch this video and 2000000 more!

SOLVED:2) Draw correct Lewis structure for tert-butyl alcohol (CH3)3COH including all non-bonding lone pairs_
- Article author: www.numerade.com
- Reviews from users: 1407
Ratings
- Top rated: 3.1
- Lowest rated: 1
- Summary of article content: Articles about SOLVED:2) Draw correct Lewis structure for tert-butyl alcohol (CH3)3COH including all non-bonding lone pairs_ Updating …
- Most searched keywords: Whether you are looking for SOLVED:2) Draw correct Lewis structure for tert-butyl alcohol (CH3)3COH including all non-bonding lone pairs_ Updating VIDEO ANSWER:to try the correct LewiS structure. We first need to count the total number of valence electrons turned beautiful alcohol has this as its chemical formula. So that means there are a total of 1234 carbons with four valence electrons. Then there is nine plus one more 10 Hydrogen with one valence electron. And then there’s one oxygen with six valence electrons. So this gives us a total of 32 valence electrons. So we’ve got a one carbon and we’ve got three Methyl groups surrounding it. Each method group has three hydrogen is coming off of it and then we have and no age. So how many electrons have we used? We’ve used 2,468, 10, 12, 14, 16, 18, 22.468, 28 oxygen doesn’t have an octet. And we’ve got four more electrons. So let’s give it two lone pairs. This will then be the complete LewiS structure for TERT, Beautiful alcohol.
- Table of Contents:
Want better grades but can’t afford to pay for Numerade
Ask your parent or guardian for help
Share Question
Add To Playlist
92% of users report better grades
Log in to watch this video and 2000000 more!

Valence electrons and ionic compounds (video) | Khan Academy
- Article author: www.khanacademy.org
- Reviews from users: 16259
Ratings
- Top rated: 4.8
- Lowest rated: 1
- Summary of article content: Articles about Valence electrons and ionic compounds (video) | Khan Academy Updating …
- Most searched keywords: Whether you are looking for Valence electrons and ionic compounds (video) | Khan Academy Updating When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic compounds.
- Table of Contents:
Valence electrons and ionic compounds
Valence electrons and ionic compounds
Video transcript
Site Navigation
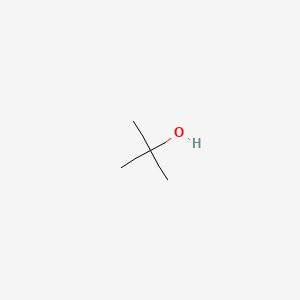
tert-Butanol | (CH3)3COH – PubChem
- Article author: pubchem.ncbi.nlm.nih.gov
- Reviews from users: 34916
Ratings
- Top rated: 4.4
- Lowest rated: 1
- Summary of article content: Articles about tert-Butanol | (CH3)3COH – PubChem tert-Butanol | (CH3)3COH or C4H10O | CID 6386 – structure, chemical names, … Tert-butyl alcohol is a colorless oily liqu with a sharp alcohol odor. …
- Most searched keywords: Whether you are looking for tert-Butanol | (CH3)3COH – PubChem tert-Butanol | (CH3)3COH or C4H10O | CID 6386 – structure, chemical names, … Tert-butyl alcohol is a colorless oily liqu with a sharp alcohol odor. tert-Butanol | (CH3)3COH or C4H10O | CID 6386 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
- Table of Contents:
brainly.com
- Article author: brainly.com
- Reviews from users: 48326
Ratings
- Top rated: 4.7
- Lowest rated: 1
- Summary of article content: Articles about brainly.com The valence electron(s) talks about the combining power of an element or compound as the case may be. Consering the t–butyl ion, … …
- Most searched keywords: Whether you are looking for brainly.com The valence electron(s) talks about the combining power of an element or compound as the case may be. Consering the t–butyl ion, …
- Table of Contents:

Modern Physical Organic Chemistry – Eric V. Anslyn, Dennis A. Dougherty – Google Sách
- Article author: books.google.com.vn
- Reviews from users: 49668
Ratings
- Top rated: 3.2
- Lowest rated: 1
- Summary of article content: Articles about Modern Physical Organic Chemistry – Eric V. Anslyn, Dennis A. Dougherty – Google Sách Updating …
- Most searched keywords: Whether you are looking for Modern Physical Organic Chemistry – Eric V. Anslyn, Dennis A. Dougherty – Google Sách Updating “The twentieth century saw the birth of physical organic chemistry – the study of the interrelationships between structure and reactivity in organic molecules – and the discipline matured to a brilliant and vibrant field. Some would argue that the last century also saw the near death of the field. In our opinion, physical organic chemistry is alive and well in the early twenty-first century. New life has been breathed into the field because it has embraced newer chemical disciplines, such as bioorganic, organometallic, materials, and supramolecular chemistries. These newer disciplines have given physical organic chemists fertile ground in which to study the interrelationships of structure and reactivity. Yet, even while these new fields have been developing, remarkable advances in our understanding of basic organic chemical reactivity have continued to appear, exploiting classical physical organic tools and developing newer experimental and computational techniques. Importantly, the techniques of physical organic chemistry and the intellectual approach to problems embodied by the discipline remain as relevant as ever to organic chemistry. Therefore, a course in physical organic chemistry will be essential for students for the foreseeable future. This book is meant to capture the state of the art of physical organic chemistry in the early twenty-first century, and, within the best of our ability, to present material that will remain relevant as the field evolves in the future. A student must know the fundamentals, such as the essence of structure and bonding in organic molecules, the nature of the basic reactive intermediates, and organic reaction mechanisms. However, students should also have an appreciation of the current issues and challenges in the field, so that when they inspect the modern literature they will have the necessary background to read and understand current research efforts. Therefore, while treating the fundamentals, we have wherever possible chosen examples and highlights from modern research areas.”–adapted from Preface, page xxiii.
- Table of Contents:
Organic Chemistry: A Mechanistic Approach – Tadashi Okuyama, Howard Maskill – Google Sách
- Article author: books.google.com.vn
- Reviews from users: 820
Ratings
- Top rated: 3.3
- Lowest rated: 1
- Summary of article content: Articles about Organic Chemistry: A Mechanistic Approach – Tadashi Okuyama, Howard Maskill – Google Sách Updating …
- Most searched keywords: Whether you are looking for Organic Chemistry: A Mechanistic Approach – Tadashi Okuyama, Howard Maskill – Google Sách Updating Organic Chemistry: A mechanistic approach provides readers with a concise review of the essential concepts underpinning the subject. It combines a focus on core topics and themes with a mechanistic approach to the explanation of the reactions it describes, making it ideal for those looking for a solid understanding of the central themes of organic chemistry. Opening with a review of chemical bonding and molecular shape and structure, the book then introduces the principal groups of organic compound before exploring the range of reactions they undergo. It retains an emphasis throughout on how and why organic compounds behave in the way they do, with a chapter on how mechanisms are investigated and the closing chapter describing the principal methods by which the structure and composition of organic compounds are studied. With an understanding of organic chemistry being central to the study and practice of a range of disciplines, Organic Chemistry is the ideal resource for those studying a one- or two-semester organic chemistry course as part of a broader programme of study in the physical and life sciences. Online Resource Centre: For registered adopters of the book: -Figures from the book in electronic format -Answers to end-of-chapter problems -Examples of organic synthesis reactions, related to topics covered in the book, for use in teaching -Additional problems (with answers), to augment those included in the book For students: -Answers to in-chapter exercises -3D-rotatable models of numerous compounds featured in the book -Multiple-choice questions for each chapter, to help students check their understanding of topics they have learned
- Table of Contents:
Organic Chemistry: A Short Course – Harold Hart, Christopher M. Hadad, Leslie E. Craine, David J. Hart – Google Sách
- Article author: books.google.com.vn
- Reviews from users: 45156
Ratings
- Top rated: 4.3
- Lowest rated: 1
- Summary of article content: Articles about Organic Chemistry: A Short Course – Harold Hart, Christopher M. Hadad, Leslie E. Craine, David J. Hart – Google Sách Updating …
- Most searched keywords: Whether you are looking for Organic Chemistry: A Short Course – Harold Hart, Christopher M. Hadad, Leslie E. Craine, David J. Hart – Google Sách Updating Offering practical, real-life applications, coverage of basic concepts, and an engaging visual style, this proven book offers a writing style, approach, and selection of topics ideal for non-chemistry science majors. This edition offers an updated, dynamic art program (online, on CD, and in the text), new content to keep you current with developments in the organic chemistry field, and a revised lab manual.Important Notice: Media content referenced within the product description or the product text may not be available in the ebook version.
- Table of Contents:
Chemistry Class 11 – [Bihar & JAC] – Dr. S. C. Rastogi, , Er. Meera Goyal – Google Sách
- Article author: books.google.com.vn
- Reviews from users: 7397
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about Chemistry Class 11 – [Bihar & JAC] – Dr. S. C. Rastogi, , Er. Meera Goyal – Google Sách Updating …
- Most searched keywords: Whether you are looking for Chemistry Class 11 – [Bihar & JAC] – Dr. S. C. Rastogi, , Er. Meera Goyal – Google Sách Updating Syllabus :Unit I : Some Basic Concepts of Chemistry, Unit II : Structure of Atom, Unit III : Classification of Elements and Periodicity in Properties,Unit IV : Chemical Bonding and Molecular Structure, Unit V : States of Matter : Gases and Liquids, Unit VI : Chemical Thermodynamics, Unit VII : Equilibrium, Unit VIII : Redox Reactions, Unit IX : Hydrogen, Unit X : s-Block Elements (Alkali and Alkaline earth metals) Group 1 and Group 2 Elements, Unit XI : Some p-Block Elements General Introduction to p-Block Elements, Unit XII : Organic Chemistry—Some Basic Principles and Techniques, Unit XIII : Hydrocarbons Classification of Hydrocarbons, Unit XIV : Environmental ChemistryContent : 1. Some Basic Concepts of Chemistry, 2. Structure of Atom, 3. Classification of Elements and Periodicity in Properties, 4. Chemical Bonding and Molecular Structure, 5. States of Matter, 6. Thermodynamics, 7. Equilibrium, 8. Redox Reactions, 9. Hydrogen, 10. s-Block Elements 11. p-Block Elements, 12. Organic Chemistry—Some Basic Principles and Techniques 13. Hydrocarbons 14. Environmental ChemistryI. AppendixII. Log-antilog Table
- Table of Contents:
See more articles in the same category here: Top 975 tips update new.
SOLVED:How many valence electrons are in the t-butyl ion (CH;) C
Video Transcript
in this question we have to tell number of valence electron in third beautiful iron. So inter tribute allow iron. We have to tell that how many valence electrons are present to attribute a lion. That is C. S. 33 C. Positive. So in this molecule we have to tell the number of valence electron present. First of all, let’s draw its structure. It has a structure of CH three. See then against CH. Three. It consists three metal group CH three CH three fine and positive charge on the central carbon. Now let’s calculate the number of bone present in this molecule. We have 123456789 10 11 12. Since this molecule consists of 12 bones and each one contributes 22 electrons, two valence electrons. So we can say that this molecule consists to help multiply by two. That means 24 valence electrons. So there are 24 valence electron in tertiary beautiful iron.
SOLVED:How many valence electrons are in the t-butyl ion (CH;) C
Video Transcript
in this question we have to tell number of valence electron in third beautiful iron. So inter tribute allow iron. We have to tell that how many valence electrons are present to attribute a lion. That is C. S. 33 C. Positive. So in this molecule we have to tell the number of valence electron present. First of all, let’s draw its structure. It has a structure of CH three. See then against CH. Three. It consists three metal group CH three CH three fine and positive charge on the central carbon. Now let’s calculate the number of bone present in this molecule. We have 123456789 10 11 12. Since this molecule consists of 12 bones and each one contributes 22 electrons, two valence electrons. So we can say that this molecule consists to help multiply by two. That means 24 valence electrons. So there are 24 valence electron in tertiary beautiful iron.
SOLVED:2) Draw correct Lewis structure for tert-butyl alcohol (CH3)3COH including all non-bonding lone pairs_
Video Transcript
to try the correct LewiS structure. We first need to count the total number of valence electrons turned beautiful alcohol has this as its chemical formula. So that means there are a total of 1234 carbons with four valence electrons. Then there is nine plus one more 10 Hydrogen with one valence electron. And then there’s one oxygen with six valence electrons. So this gives us a total of 32 valence electrons. So we’ve got a one carbon and we’ve got three Methyl groups surrounding it. Each method group has three hydrogen is coming off of it and then we have and no age. So how many electrons have we used? We’ve used 2,468, 10, 12, 14, 16, 18, 22.468, 28 oxygen doesn’t have an octet. And we’ve got four more electrons. So let’s give it two lone pairs. This will then be the complete LewiS structure for TERT, Beautiful alcohol.
So you have finished reading the how many valence electrons are in the t-butyl ion ch33c+ topic article, if you find this article useful, please share it. Thank you very much. See more: how many valence electrons are in the t-butanol molecule ch33coh?, how many valence electrons are in the methane molecule ch4, how many valence electrons are in the peroxide ion, how many valence electrons are in the ethanol molecule ch3ch2oh, how many valence electrons are in the nitric acid molecule, how many valence electrons are in the sulfuric acid molecule h2so4

