You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how many water molecules could hydrogen bond directly to glucose on Google, you do not find the information you need! Here are the best content compiled and compiled by the Chewathai27.com team, along with other related topics such as: how many water molecules could hydrogen bond directly to glucose how many hydrogen bonds can a water molecule form, a hydrogen atom in a given water molecule is strongly attracted to, how many hydrogen bonds can hf form, how many hydrogen bonds can nh3 form, hydrogen bonding in water and ice, how does hydrogen bonding occur between water molecules, how many hydrogen bonds can ethanol form, bond between oxygen and hydrogen in a water molecule
Contents
How many hydrogen bond attractions can a molecule of glucose form with water?
Remembering our definition of hydrogen bond donors and acceptors, you can see that glucose has 5 hydrogen bond donors (H-O) and 6 hydrogen bond acceptors (O). Water molecules can form hydrogen bonds at each of these sites and solvate the glucose molecule.
How many water molecules could hydrogen bond directly to the molecules of glucose sorbitol and Ribitol?
Glucose =17, sorbitol =18, ribitol =15; each alcohol group can bond to three water molecules and the ring oxygen binds to two.
Does water hydrogen bond with glucose?
Glucose is a highly polar molecule having six hydroxyl groups in its structure. Due to the dipole moment of water, glucose form hydrogen bond with water and hydrogen enthalpy of glucose is sufficient to make it soluble in water [36] .
How many water molecules are needed for glucose?
Twenty-four
Was this answer helpful?
How many hydrogen bonds can each water molecule form?
Each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water molecules. These four hydrogen bonds optimally arrange themselves tetrahedrally around each water molecule as found in ordinary ice (see right).
Can glucose form hydrogen bonds?
The hydroxyl groups in glucose molecules can form strong hydrogen bonds with the solvent (water) molecules, so glucose is soluble in water.
How many hydrogen bonds can lactic acid form?
3.1. Lactic and Malic Acids Have Double Hydrogen Bonds between Carboxyl Groups in Moderately Acidic Solutions.
Which molecule could form hydrogen bonds quizlet?
-Hydrogen bonding occurs only in molecules where hydrogen is covalently bonded to one of three elements: fluorine, oxygen, or nitrogen.
What are the requirements for molecules to form hydrogen bond what atoms must be present and involved in such bonds ?)?
In order for a hydrogen bond to occur there must be both a hydrogen donor and an acceptor present. The donor in a hydrogen bond is usually a strongly electronegative atom such as N, O, or F that is covalently bonded to a hydrogen bond.
How many hydrogen bonds are in fructose?
Fructose is another sugar that also has 6 carbons, 12 hydrogens, and 6 oxygen atoms.
How many water molecules are present in sucrose?
6 water molecules are present in sucrose.
What is the bond between water and sugar?
For a liquid to dissolve a solid, the molecules of the liquid and solid must attract one another. The bond between the oxygen and hydrogen atoms (O–H bond) in sugar (sucrose) gives the oxygen a slight negative charge and the hydrogen a slight positive charge. Sucrose is a polar molecule.
How many water molecules are required for photosynthesis?
Notice that it takes six molecules of water and six molecules of carbon dioxide to make one molecule of glucose. The inputs and outputs of photosynthesis are shown in the diagram at right. Carbon dioxide in the air enters a plant through its leaves.
How many water molecules are involved in photosynthesis process?
during the process of photosynthesis molecules of water are splitted.. During the photosynthetic reaction water is produced from the oxygen atoms in the carbon dioxide molecule. six molecule of water and six molecule of carbon dioxide react in the presence of sunlight to form 1 glucose and 6 moles of oxygen.
Why is there a 6 in the photosynthesis equation?
During photosynthesis, carbon dioxide and water are converted into glucose and oxygen. Glucose is a large molecule with 6 carbon molecules (C6H12O6), therefore the smallest number of carbon dioxide (CO2) molecules required to make one molecule of glucose is 6!
What intermolecular forces are present in glucose?
Intermolecular forces
*glucose is a monosaccharides. The intermolecular forces glucose has are hydrogen bonding between the hydrogen and oxygen, dipole dipole between the negative end and positive end of the molecule and London dispersion forces, which all molecules have.
Why is glucose soluble in water?
Glucose is small (6 carbons) and dissolves easily in water because it has a number of polar OH groups attached to its carbons. Glucose (and other things we’ll talk about later) is taken up in your intestine from your food and transported in your blood so that the many cells of your body can use it.
Is glucose soluble in water?
Is glucose hydrophobic or hydrophilic?
Glucose, a monosaccharide
Each of the carbon atoms is also joined to at least one hydrogen atom and to one oxygen atom. The presence of all this oxygen in the structure of the glucose molecule ensures that it is strongly hydrophilic (‘loves’ water).
Lecture 5
- Article author: butane.chem.uiuc.edu
- Reviews from users: 27371
Ratings
- Top rated: 3.6
- Lowest rated: 1
- Summary of article content: Articles about Lecture 5 Updating …
- Most searched keywords: Whether you are looking for Lecture 5 Updating
- Table of Contents:

SOLVED:How many water molecules could hydrogenbond directly to the molecules of glucose, sorbitol, and ribitol, shown here?
- Article author: www.numerade.com
- Reviews from users: 2608
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about SOLVED:How many water molecules could hydrogenbond directly to the molecules of glucose, sorbitol, and ribitol, shown here? Updating …
- Most searched keywords: Whether you are looking for SOLVED:How many water molecules could hydrogenbond directly to the molecules of glucose, sorbitol, and ribitol, shown here? Updating VIDEO ANSWER: Problems 17 of Chapter two. Water, the solvent of biochemical reactions. So in this question, the number of water molecule state could hydrogen bond to glucose, sorbitol and rip it out. So here, the cMath Solutions, Chemistry 102, , Chapter
- Table of Contents:
Glucose $=17$ sorbitol $=18$ ribitol $=15 ;$ each alcohol group can bond to three water molecules and the ring oxygen binds to two The sugar alcohols bind more than the corresponding sugars
Add To Playlist
Share Question
Report Question

Attention Required! | Cloudflare
- Article author: www.toppr.com
- Reviews from users: 8276
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about Attention Required! | Cloudflare Updating …
- Most searched keywords: Whether you are looking for Attention Required! | Cloudflare Updating
- Table of Contents:
Please complete the security check to access wwwtopprcom
Why do I have to complete a CAPTCHA
What can I do to prevent this in the future

Access to this page has been denied.
- Article author: www.chegg.com
- Reviews from users: 1737
Ratings
- Top rated: 4.9
- Lowest rated: 1
- Summary of article content: Articles about Access to this page has been denied. Hydrogen Bonds. REFLECT AND APPLY How many water molecules could hydrogenbond directly to the molecules of glucose, sorbitol, and ribitol, shown here? …
- Most searched keywords: Whether you are looking for Access to this page has been denied. Hydrogen Bonds. REFLECT AND APPLY How many water molecules could hydrogenbond directly to the molecules of glucose, sorbitol, and ribitol, shown here?
- Table of Contents:

Hydrogen bond dynamics and water structure in glucose-water solutions by depolarized Rayleigh scattering and low-frequency Raman spectroscopy: The Journal of Chemical Physics: Vol 127, No 2
- Article author: aip.scitation.org
- Reviews from users: 25053
Ratings
- Top rated: 5.0
- Lowest rated: 1
- Summary of article content: Articles about Hydrogen bond dynamics and water structure in glucose-water solutions by depolarized Rayleigh scattering and low-frequency Raman spectroscopy: The Journal of Chemical Physics: Vol 127, No 2 Thus the glucose imposes a new local order among water molecules localized in its hydration shell in which the hydrogen bond breaking dynamics is … …
- Most searched keywords: Whether you are looking for Hydrogen bond dynamics and water structure in glucose-water solutions by depolarized Rayleigh scattering and low-frequency Raman spectroscopy: The Journal of Chemical Physics: Vol 127, No 2 Thus the glucose imposes a new local order among water molecules localized in its hydration shell in which the hydrogen bond breaking dynamics is … The effect of glucose on the relaxation process of water at picosecond time scales has been investigated by depolarized Rayleigh scattering (DRS) experiments. The process is assigned to the fast hy…
- Table of Contents:

Hydrogen bonds in water (article) | Khan Academy
- Article author: www.khanacademy.org
- Reviews from users: 8780
Ratings
- Top rated: 4.6
- Lowest rated: 1
- Summary of article content: Articles about Hydrogen bonds in water (article) | Khan Academy Below, we’ll look at how this hydrogen bonding works. Polarity of water molecules. The key to understanding water’s chemical behavior is its molecular structure … …
- Most searched keywords: Whether you are looking for Hydrogen bonds in water (article) | Khan Academy Below, we’ll look at how this hydrogen bonding works. Polarity of water molecules. The key to understanding water’s chemical behavior is its molecular structure … The structure of water molecules and how they can interact to form hydrogen bonds.
- Table of Contents:
Structure of water and hydrogen bonding
Structure of water and hydrogen bonding
Site Navigation
See more articles in the same category here: Top 975 tips update new.
Lecture 5
Lecture 5
Solution Chemistry
Now that we have looked at how to balance reactions, we can study chemical reactions in more detail. A solution is a homogeneous mixture of two or more substance. The solute is the substance present in the smaller amount and the solvent is the substance present in the larger amount. Solutions can be:
gaseous – air is a solution of N 2 and O 2 , with traces of Ar, CO 2 , Ne, He, Kr and Xe
solid – metal alloys are solutions – steel is Fe with a small amount of C
liquid – seawater is a solution of H 2 O, NaCl and other trace ions
Many important, and virtually all biological processes take place in aqueous solutions, in the most common and useful solvent, water.
Water
Water is a covalent molecule, formed by the two non-metals, hydrogen and oxygen. Because of the spatial distribution of the valence electrons on oxygen, water was the shape shown below.
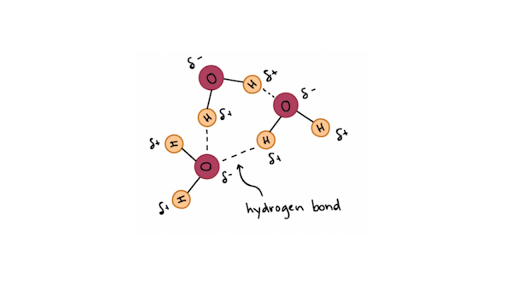
It is not a linear molecule, but has a bent shape. The central oxygen atom (red) is sharing its six valence electrons with the two hydrogen atoms (white), each with one valence electron. However, the electrons are not being shared equally between the oxygen and hydrogens. Oxygen attracts electrons much more strongly than hydrogen, which leads to the formation of partial electrical charges within the water molecule. The picture, below, represents the size and shape of the electron cloud in a water molecule. It is color coded to indicate charge. The oxygen atom actually carries a net negative charge, since the electrons spend more time in the vicinity of the oxygen. The hydrogen atoms carry a net positive charge.
The separation of charge within a molecule is called a dipole. There is a negative side to the molecule and a positive side. This is shown on the figure below by the addition of the “δ” symbol. This indicates partial positive ( δ+) or partial negative (δ-) charge. The arrow next to the molecule represents the dipole moment of the molecule, indicating the direction of the charge imbalance.
This makes water a polar solvent. Remember that opposite charges attract. This means that water molecules will interact with each other. The positively charged hydrogens in one water molecule will be attracted to the negatively charged oxygens on other water molecules. This association is called hydrogen bonding. One component of a hydrogen bond is called a hydrogen bond donor and is a hydrogen with a partial positive charge (δ+). This occurs when hydrogen is bonded to oxygen, nitrogen or fluorine, i.e, when you have H-O, H-N or H-F in a molecule. The second component of a hydrogen bond is called a hydrogen bond acceptor. These are O, or N or F atoms in a compound. A representation of water molecules hydrogen bonded to each other is shown below. The purple lines represent the hydrogen bonds between water molecules. The distance between the atoms, and the bond angle between the water molecules are also shown.
Solubility of Salts
So, even in the liquid state, water molecules are highly organized. When we make an aqueous solution we are using water as the solvent and some other chemical species as the solute. An example of this would be a saltwater solution, involving H 2 O and NaCl. You know that if you add table salt to water it will dissolve to form a solution. The structure of the salt, sodium chloride is shown below.
In NaCl, the attraction is between Na+, the sodium cation, and Cl-, the chloride anion. This is an ion-ion interaction, involving full positive and negative charges. When NaCl dissolves in water, the ion-ion interactions are replaced by ion-dipole interactions. The picture below illustrates the formation of ion-dipole interactions between the Na+ ions (purple) and the Oδ- oxygens of water (red) and between the Cl- ions (yellow) and the Hδ+ hydrogens of water (white). There are still hydrogen bonds between many of the water molecules. The Na+ and Cl- are said to be solvated i.e., surrounded by solvent (H 2 O) molecules.
This reaction would be written as:
NaCl (s) + H 2 O (l) → Na+ (aq) + Cl- (aq)
The solubility of ionic substances in water varies greatly. It depends on the relative strength of the ion-ion interaction in the solid state and the ion-dipole interaction in solution. We will discuss this in more detail in the next lecture.
Solubility of non-ionic Compounds
Some non-ionic substances are also soluble in water. For example, glucose, C 6 H 12 O 6 . The grey atoms are carbon, the red atoms, oxygen and the white atoms, hydrogen. Remembering our definition of hydrogen bond donors and acceptors, you can see that glucose has 5 hydrogen bond donors (H-O) and 6 hydrogen bond acceptors (O). Water molecules can form hydrogen bonds at each of these sites and solvate the glucose molecule.
C 6 H 12 O 6 (s) + H 2 O → C 6 H 12 O 6 (aq)
There are some molecules, which are completely insoluble in water. One example is octane, a component of gasoline. It has the formula C 8 H 18 and the structure shown below:
It has no hydrogen bond donors (hydrogens bonded to O, N or F) and it has no hydrogen bond acceptors (O, N or F). So, it cannot form hydrogen bonds to water. It is completely insoluble in water. It is a non-polar compound, which has no separation of charge within the compound. Since it has no full or partial charges, it cannot interact with species which do carry charges. This is the rule in solubility, “Like dissolves like”. Charged or polar substances dissolve in polar solvents. Non-charged substances dissolve in non-polar solvents.
Properties of Aqueous Solutions
There is a fundamental difference between aqueous solutions of ionic substances (like NaCl) and covalent substances (like sugar). Ionic substances conduct electricity and are called electrolytes. Electrolytes are substances whose aqueous solutions conduct electricity because they contain mobile, charge carrying ions.
Strong electrolytes are substances that are completely ionized when dissolved in water.
Weak electrolytes are substances that are only partly ionized in water.
Non-electrolytes are substances which dissolve in water but which produce no ions.
Electrolytes can be further subdivided into three categories, acids, bases and salts.
Acids have a sharp, sour taste, turn litmus paper red and produce H+ in aqueous solutions.
Bases have a bitter taste, turn litmus paper blue and produce OH- in aqueous solutions.
Salts are ionic compounds, which produce a cation other than H+ and an anion other than OH- in solution.
Here are a set of rules, and their exceptions to use in defining electrolytes:
Rule Exceptions
1. Most acids are weak electrolytes HCl, HI, HBr
HNO 3 , HClO 4 , H 2 SO 4
2. Most bases are weak electrolytes LiOH – CsOH
Ca(OH) 2 – Ba(OH) 2
3. Most salts are strong electrolytes HgCl 2 , Hg(CN) 2
Strong Electrolytes
These are the species, which dissociate completely in water, to give a solution of hydrated anions and cations.
strong acids
HCl → H+ (aq) + Cl- (aq)
The single arrow (→) indicates that the reaction goes to completion, that all of the HCl is converted to ions, H+ and Cl-.
strong bases
NaOH (s) → Na+ (aq) + OH- (aq)
salts
MgSO 4 (s) → Mg2+ (aq) + SO 4 – (aq)
Sulfuric acid, a strong acid, dissociates as shown below:
H 2 SO 4 → H+ (aq) + HSO 4 – (aq)
only one of the two acidic H+ dissociate completely.
Weak Electrolytes
These are the species which undergo partial dissociation in aqueous solutions.
weak acids
HC 2 H 3 O 2 (aq) D H+ (aq) + C 2 H 3 O 2 – (aq)
The double arrow (D) indicates that there is an incomplete reaction, and that all of the species, HC 2 H 3 O 2 (acetic acid), H+ and C 2 H 3 O 2 – (acetate), will be present in the solution.
weak bases
NH 3 (aq) + H 2 O (l) D NH 4 +(aq) + OH- (aq)
weak electrolytic salt
HgCl 2 (aq) D Hg2+ (aq) + 2 Cl- (aq)
HgCl 2 dissolves and exists as a “molecular” unit in the solution, along with Hg2+ and Cl-.
Non-Electrolytes
These are species which dissolve in water, but which produce no ions.
C 2 H 5 OH (l) + H 2 O (l) → C 2 H 5 OH (aq)
This equation describes the mixing of ethanol (drinking alcohol) in water. Since ethanol has a hydrogen bond donor (O-H) and a hydrogen bond acceptor (O) it will form hydrogen bonds to water and the two liquids will dissolve in each other. No ions are formed in this process.
Solution Composition
Solutions are usually described in terms of their concentration which is the amount of solute dissolved in a given volume of solvent. One measure of concentration is molarity.
Let’s look at different uses of this equation:
1. We could calculate the molarity of a solution made by dissolving 23.4 g of sodium sulfate in enough water to form 125 mL of solution.
Na 2 SO 4 (s) + H 2 O → 2 Na+ (aq) + SO 4 2- (aq)
Molarity = moles Na 2 SO 4
liters of solution
23.4 g Na 2 SO 4 1 mol Na 2 SO 4 = 0.165 mol Na 2 SO 4
142 g Na 2 SO 4
125 mL 1 L = 0.125 L
1000 mL
Molarity = 1.165 mole Na 2 SO 4 = 1.32 M Na 2 SO 4
0.125 L
2. We could calculate the number of moles of HNO 2 in 2.0 L of 0.200 M HNO 3 .
0.200 M HNO 3 = 0.200 mol HNO 3 2.0 L = 0.40 mol
1 L
3. How many grams of Na 2 SO 4 are required to make 350 mL of 0.500 M NaSO 4 ?
0.500 mol 1 L 350 mL 142 g Na 2 SO 4 = 24.9 g Na 2 SO 4
1 L 1000 mL mol Na 2 SO 4
Dilution
A stock solution is a concentrated solution of known composition. For example, hydrochoric acid (HCl) is usually purchased as 12 M HCl.
Less concentrated solutions can be made by diluting (adding water) to this stock solution.
moles of solute before dilution = moles of solute after dilution
How could we prepare 250 mL of 0.1 M CuSO 4 , by diluting a 1.0 M stock solution of CuSO 4 ?
First, figure out moles of solute after dilution.
final volume = 250 x 10-3 L = 250 mL
final concentration = 0.1 M = 0.1 mol/L
final moles of CuSO 4 = 1.0 mol 250 x 10-3 L = 25 x 10-3 mol CuSO 4
1 L
So, we will need to start with 25 x 10-3 mol CuSO 4 .
25 x 10-3 mol CuSO 4 1 L = 25 x 10-3 L of the 1.0 M stock solution
1.0 mol
So, we would take 25 mL of the 1.0 M stock solution, and add 100 mL of water to bring us to the final volume of 125 mL.
(M initial )(V initial ) = (M final )(V final )
SOLVED:How many water molecules could hydrogenbond directly to the molecules of glucose, sorbitol, and ribitol, shown here?
Video Transcript
Problems 17 of Chapter two. Water, the solvent of biochemical reactions. So in this question, the number of water molecule state could hydrogen bond to glucose, sorbitol and rip it out. So here, the concept behind the question is the hydrogen born that originate because of electrostatic attractions. The hydrogen bond is also considered as unique case of type of type of interaction for the formation of hydrogen bond hydrogen atom as well as electoral negative atom like oxygen nitrogen item that is required. So the number of water molecules that could hydrogen born directly to following molecule is as follow. For example, in case of close calls, so in case of glucose, each O. H group can hydrogen bond with three water molecules and the oxygen in the ring can bond with two water molecules. So glucose has 50 H group, That is three into five. That is equal to 15 water molecules and one oxygen in the ring. That meant to water molecules. So therefore glucose have total 17. So glucose has total 17 number of water molecules that could hydrogen bond to glucose. So in case of sorbitol, so sorbitol has 60 H group. So I can write this three into six, which means 18 water molecules. So therefore for sorbitol, that is a call to 18. And in case of rebuttal, rebuttal has 50. H group. So I can write three into five. That is a gold 015 water molecules. And therefore in case of uh glucose we have 17. in case of sorbitol we have 18 and in case of rebuttal we have 15 water molecule that could hydrogen bond directly to the following molecules for glucose, it is 17 for sorbitol, That is 18, and rebuttal that is 15.
So you have finished reading the how many water molecules could hydrogen bond directly to glucose topic article, if you find this article useful, please share it. Thank you very much. See more: how many hydrogen bonds can a water molecule form, a hydrogen atom in a given water molecule is strongly attracted to, how many hydrogen bonds can hf form, how many hydrogen bonds can nh3 form, hydrogen bonding in water and ice, how does hydrogen bonding occur between water molecules, how many hydrogen bonds can ethanol form, bond between oxygen and hydrogen in a water molecule

