You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how many atoms are in 5.6 moles of na on Google, you do not find the information you need! Here are the best content compiled and compiled by the https://chewathai27.com team, along with other related topics such as: how many atoms are in 5.6 moles of na
Contents
What is the number of atoms in a mole of Na?
ONE MOLE of any element has 6.023×1023 atoms of that element. Therefore 1 mole or 22.99 grams of Na = 6.023×1023 atoms of Na.
How many atoms are in 5 moles?
Hence, number of moles of Oxygen in 5 Moles of CO = 5. The number of oxygen atoms present can be obtained by multiplying the moles of oxygen by Avogadro’s Number. So, number of oxygen atoms present = 5 x ( 6.023 x 10^23 ) = 30.115 x 10^23 nos.
How many atoms are in 4 moles Na?
2.4×1024 atoms.
How many atoms are there in 1.5 mol of Na?
► 1.50 moles of sodium is how many atoms? answers! ► 9.03 x 1023 atoms Na Page 24 ► For BOTH mass and particles it is ► ALWAYS ONE MOLE!
How many atoms does Na?
| Element | Average Atomic Mass (amu) | Atoms/Mole |
|---|---|---|
| C | 12.01 | 6.022×1023 |
| H | 1.008 | 6.022×1023 |
| O | 16.00 | 6.022×1023 |
| Na | 22.99 | 6.022×1023 |
How many atoms are in 2 moles Na?
If we have 2 mol of Na atoms, we have 2 × (6.022 × 10 23) Na atoms, or 1.2044 × 10 24 Na atoms.
How many atoms are there in 5 moles of sodium atom?
As, 1 mole = 6.022 x 1023 atoms (b) Here, both containers have 5 moles of each carbon and sodium, therefore, both containers have equal number of atoms, i.e., 5 x 6.022 x 10 atoms. or 3.011 x 1024 atoms in each.
How many atoms are in a mole?
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro’s Number (6.0221421 x 1023).
What is the mass of 5 moles of sodium?
Mass of sodium is 23 grams. So the total mass of 5 moles of sodium will be 5×23=165 .
How do you calculate atoms?
To calculate the number of atoms in a sample, divide its weight in grams by the amu atomic mass from the periodic table, then multiply the result by Avogadro’s number: 6.02 x 10^23.
How do you convert moles to atoms?
Converting moles to atoms is as simple as multiplying the number of moles by the 6.022 * 10^23 because by definition that is what a mole represents.
How many grams are in Na?
And whereas one sodium atom has an approximate mass of 23 u, 1 mol of Na atoms has an approximate mass of 23 grams. One mole of a substance has the same mass in grams that one atom or molecule has in atomic mass units.
How many atoms are in 4.5 moles?
A mole of anything has 6.022 x 1023 items in it. 4.5 moles of copper has (4.5)(6.022 x 1023) = 2.7 x 1024 atoms.
What is the mass of 1.5 mole of Na ions?
Explanation: 1 mole is equal to 1 moles Na, or 22.98977 grams.
What is the mass of 2.5 moles of sodium?
And since we know that 1⋅mol of sodium has a mass of 22.99⋅g , we got a mass of 2.50⋅mol×22.99⋅g⋅mol−1=57.5⋅g with respect ot sodium.
How many atoms are in 1 mole of atoms?
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro’s Number (6.0221421 x 1023).
What is the number of atoms in a mole of any element?
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant.
What is the molar mass of Na?
brainly.com
- Article author: brainly.com
- Reviews from users: 47283
Ratings
- Top rated: 4.8
- Lowest rated: 1
- Summary of article content: Articles about brainly.com 2. How many atoms are in 5.6 moles of Na? Answer:___3.2 E 24 atoms of Na. …
- Most searched keywords: Whether you are looking for brainly.com 2. How many atoms are in 5.6 moles of Na? Answer:___3.2 E 24 atoms of Na.
- Table of Contents:

how many atoms are in 5.6 moles of na
- Article author: static1.squarespace.com
- Reviews from users: 8455
Ratings
- Top rated: 4.8
- Lowest rated: 1
- Summary of article content: Articles about how many atoms are in 5.6 moles of na Updating …
- Most searched keywords: Whether you are looking for how many atoms are in 5.6 moles of na Updating
- Table of Contents:

Calculate the number of moles of oxygen atoms in 5 moles of CO ?
- Article author: www.careers360.com
- Reviews from users: 31178
Ratings
- Top rated: 4.4
- Lowest rated: 1
- Summary of article content: Articles about Calculate the number of moles of oxygen atoms in 5 moles of CO ? Updating …
- Most searched keywords: Whether you are looking for Calculate the number of moles of oxygen atoms in 5 moles of CO ? Updating
- Table of Contents:
Related Questions
Calculate the number of moles of oxygen Arindom 5 moles of CO
calculate total number of moles of atoms present in 49g H2SO4
How many oxygen atoms are present in 10 moles of Carbon – di -oxide
How many moles of magnesium phosphate will contain 025 mole of Oxygen atoms
calculate oxidation number of Co in { CoBr2(CN)2}+
Download the Careers360 App on your Android phone

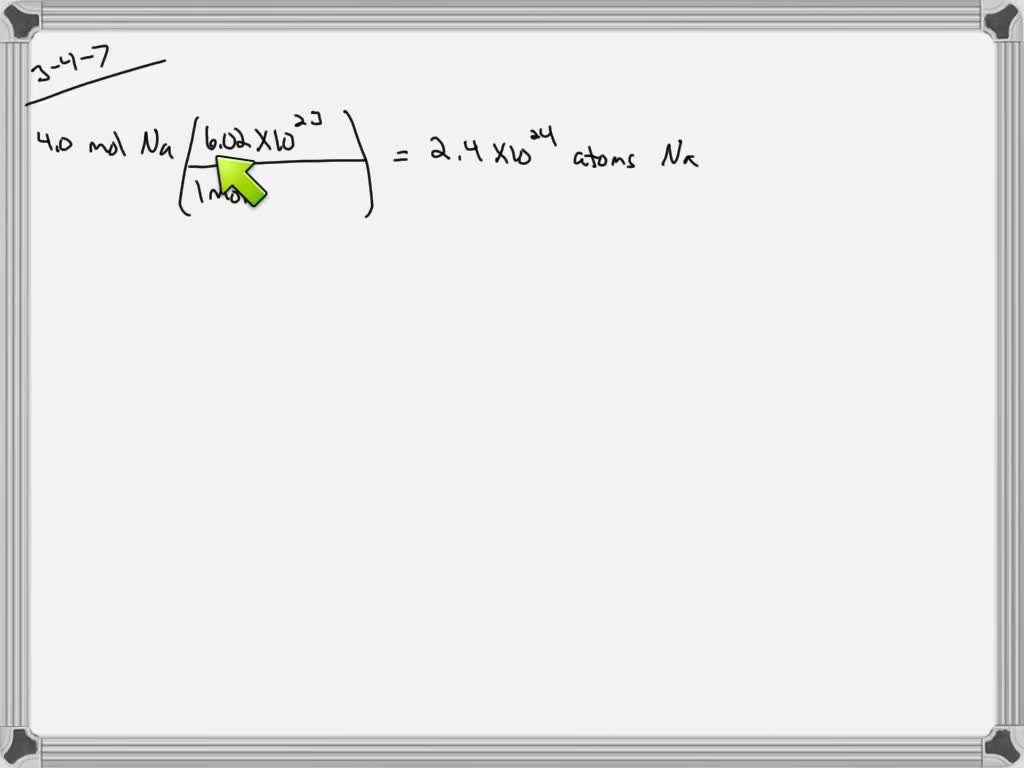
SOLVED:How many atoms are present in 4.0 mol of sodium?
- Article author: www.numerade.com
- Reviews from users: 35371
Ratings
- Top rated: 4.9
- Lowest rated: 1
- Summary of article content: Articles about SOLVED:How many atoms are present in 4.0 mol of sodium? Updating …
- Most searched keywords: Whether you are looking for SOLVED:How many atoms are present in 4.0 mol of sodium? Updating VIDEO ANSWER: if we have 4.0 moles of sodium, that all we need to do to get the number of sodium atoms is multiplied by avocados number, which is 6.2 times 10 of the 23rd and 22 significant figures as represented hMath Solutions, Chemistry 101, , Chapter
- Table of Contents:
$24 times 10^{24}$ atoms
Add To Playlist
Share Question
Report Question
how many atoms are in 5.6 moles of na
- Article author: www.usd497.org
- Reviews from users: 23336
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about how many atoms are in 5.6 moles of na Updating …
- Most searched keywords: Whether you are looking for how many atoms are in 5.6 moles of na Updating
- Table of Contents:

2. How many atoms are in 5.6 moles of Na? Answer:___3… – Techwhiff
- Article author: www.techwhiff.com
- Reviews from users: 17204
Ratings
- Top rated: 4.5
- Lowest rated: 1
- Summary of article content: Articles about 2. How many atoms are in 5.6 moles of Na? Answer:___3… – Techwhiff Answer:5.66.02∗10231 = 3.4 X 1024Explanation:the correct answer to it is 3.4 instead of 3.2. I got 3.4 from rounding it from 3.37. …
- Most searched keywords: Whether you are looking for 2. How many atoms are in 5.6 moles of Na? Answer:___3… – Techwhiff Answer:5.66.02∗10231 = 3.4 X 1024Explanation:the correct answer to it is 3.4 instead of 3.2. I got 3.4 from rounding it from 3.37.
- Table of Contents:

2. How many atoms are in 5.6 moles of Na? ___3.2 E 24 at… – LTWork
- Article author: ltwork.net
- Reviews from users: 26250
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about 2. How many atoms are in 5.6 moles of Na? ___3.2 E 24 at… – LTWork Explanation: 1) How many moles are 1.55 × 10²⁷ atoms of zinc? 0.26 × 10⁴ mole. Explanation: The given problem will solve by using Avogadro number. …
- Most searched keywords: Whether you are looking for 2. How many atoms are in 5.6 moles of Na? ___3.2 E 24 at… – LTWork Explanation: 1) How many moles are 1.55 × 10²⁷ atoms of zinc? 0.26 × 10⁴ mole. Explanation: The given problem will solve by using Avogadro number.
- Table of Contents:

Error 403 (Forbidden)
- Article author: www.quora.com
- Reviews from users: 8336
Ratings
- Top rated: 4.6
- Lowest rated: 1
- Summary of article content: Articles about Error 403 (Forbidden) Therefore 5.6 grams of urea would contain ( 5.6 X 4 X Avogadro number ) / 60 atoms. …
- Most searched keywords: Whether you are looking for Error 403 (Forbidden) Therefore 5.6 grams of urea would contain ( 5.6 X 4 X Avogadro number ) / 60 atoms.
- Table of Contents:

how many atoms are in 5.6 moles of na
- Article author: www.livingston.org
- Reviews from users: 21217
Ratings
- Top rated: 4.3
- Lowest rated: 1
- Summary of article content: Articles about how many atoms are in 5.6 moles of na Molar Mass: the mass of one mole of an element. CONVERSION FACTORS: 1 mole = 6.02 x 10. 23 atoms 1 mole = atomic mass (g). Try: 1. How many atoms are in 6.5 … …
- Most searched keywords: Whether you are looking for how many atoms are in 5.6 moles of na Molar Mass: the mass of one mole of an element. CONVERSION FACTORS: 1 mole = 6.02 x 10. 23 atoms 1 mole = atomic mass (g). Try: 1. How many atoms are in 6.5 …
- Table of Contents:

how many atoms are in 5.6 moles of na
- Article author: www.montgomerycollege.edu
- Reviews from users: 9043
Ratings
- Top rated: 3.5
- Lowest rated: 1
- Summary of article content: Articles about how many atoms are in 5.6 moles of na Calculate the number of atoms in 2.45 mol of copper. A silver ring contains 1.1 x 1022 silver atoms. How many moles of silver are in the ring? …
- Most searched keywords: Whether you are looking for how many atoms are in 5.6 moles of na Calculate the number of atoms in 2.45 mol of copper. A silver ring contains 1.1 x 1022 silver atoms. How many moles of silver are in the ring?
- Table of Contents:

Calculate following in 5.6g of nitrogena) number of moles of N2b)number of molecules of N2c)number of atoms of nitrogen – Chemistry Q&A
- Article author: byjus.com
- Reviews from users: 26131
Ratings
- Top rated: 3.7
- Lowest rated: 1
- Summary of article content: Articles about Calculate following in 5.6g of nitrogena) number of moles of N2b)number of molecules of N2c)number of atoms of nitrogen – Chemistry Q&A =0.2 mol. b) No.of molecules of N2 in 5.6g of nitrogen … Why does sodium keep immersed in kerosene oil? … Write any monomer used in terylene? …
- Most searched keywords: Whether you are looking for Calculate following in 5.6g of nitrogena) number of moles of N2b)number of molecules of N2c)number of atoms of nitrogen – Chemistry Q&A =0.2 mol. b) No.of molecules of N2 in 5.6g of nitrogen … Why does sodium keep immersed in kerosene oil? … Write any monomer used in terylene? Get a comprehensive answer to this question and access a vast question bank that is tailored for students. Access detailed answers to interesting Science & Maths questions at BYJU’S.
- Table of Contents:
Attention Required! | Cloudflare
- Article author: www.toppr.com
- Reviews from users: 44256
Ratings
- Top rated: 4.9
- Lowest rated: 1
- Summary of article content: Articles about Attention Required! | Cloudflare Click here to get an answer to your question ✍️ Calculate the number of atoms of hydrogen present in 5.6g of urea (molar mass of urea = 60g mol^-1 ). …
- Most searched keywords: Whether you are looking for Attention Required! | Cloudflare Click here to get an answer to your question ✍️ Calculate the number of atoms of hydrogen present in 5.6g of urea (molar mass of urea = 60g mol^-1 ).
- Table of Contents:
Please complete the security check to access wwwtopprcom
Why do I have to complete a CAPTCHA
What can I do to prevent this in the future

See more articles in the same category here: https://chewathai27.com/toplist.
2. How many atoms are in 5.6 moles of Na? ___3.2 E 24 at…
Explanation:
1) How many moles are 1.55 × 10²⁷ atoms of zinc?
0.26 × 10⁴ mole
Explanation:
The given problem will solve by using Avogadro number.
It is the number of atoms , ions and molecules in one gram atom of element, one gram molecules of compound and one gram ions of a substance.
The number 6.022 × 10²³ is called Avogadro number.
For example,
18 g of water = 1 mole = 6.022 × 10²³ molecules of water
1.008 g of hydrogen = 1 mole = 6.022 × 10²³ atoms of hydrogen
For 1.55 × 10²⁷ atoms of zinc:
1.55 × 10²⁷ atoms × 1 mole/ 6.022 × 10²³ atoms
0.26 × 10⁴ mole
2) How many atoms are in 25.0 moles of calcium?
150.55 × 10²³ atoms.
Explanation:
According to Avogadro number,
one mole of substance = 6.022 × 10²³ atoms
The number 6.022 × 10²³ is called Avogadro number.
For 25.0 moles of calcium:
one mole of calcium = 6.022 × 10²³ atoms
25.0 mole × 6.022 × 10²³ atoms /1 mole
150.55 × 10²³ atoms.
So 25.0 moles of calcium contain 150.55 × 10²³ atoms.
3) = How many atoms are in 0.35 moles of carbon?
2.12× 10²³ atoms.
Explanation:
According to Avogadro number,
one mole of substance = 6.022 × 10²³ atoms
The number 6.022 × 10²³ is called Avogadro number.
For 0.35 moles of carbon:
one mole of carbon = 6.022 × 10²³ atoms
0.35 mole × 6.022 × 10²³ atoms /1 mole
2.12× 10²³ atoms.
So 0.35 moles of carbon contain 2.12× 10²³ atoms.
4 ) How many moles are 7.6 × 10²⁴ atoms of hellium?
The given problem will solve by using Avogadro number.
It is the number of atoms , ions and molecules in one gram atom of element, one gram molecules of compound and one gram ions of a substance.
The number 6.022 × 10²³ is called Avogadro number.
For example,
18 g of water = 1 mole = 6.022 × 10²³ molecules of water
1.008 g of hydrogen = 1 mole = 6.022 × 10²³ atoms of hydrogen
For 7.6 × 10²⁴ atoms of hellium:
7.6 × 10²⁴ atoms × 1 mole/ 6.022 × 10²³ atoms
12. 6 mole
Mass – Mole conversion:
How many moles in 28.0 gram of oxygen?
1.75 mol
Explanation:
Given data:
Mass of oxygen = 28 g
Number of moles = ?
Solution:
Formula:
Number of moles = mass/ molar mass
Molar mass of oxygen = 16 g/mol
Now we will put the values in formula:
Number of moles = 28 g / 16 g/mol
Number of moles = 1.75 mol
So 28 g of oxygen contain 1.75 moles.
3) Find the number of moles of argon in 452 g?
11.31 mol
Explanation:
Given data:
Mass of argon = 452 g
Number of moles = ?
Solution:
Formula:
Number of moles = mass/ molar mass
Molar mass of argon= 39.95 g/mol
Now we will put the values in formula:
Number of moles = 452 g /39.95 g/mol
Number of moles = 11.31 mol
So 452 g of argon contain 11.31 moles.
2) What is the mass of 5.0 moles of iron?
Mass = 279.25 g
Explanation:
Given data:
Mass of iron = ?
Number of moles of iron = 5.0 mol
Solution:
Formula:
Mass = number of moles × molar mass
Molar mass of iron = 55.85 g/mol
Now we will put the values in formula:
Mass = number of moles × molar mass
Mass = 5 mol × 55.85 g/mol
Mass = 279.25 g
4) Find the grams of 16.5 mole of hydrogen?
Mass = 16.632 g
Explanation:
Given data:
Mass of hydrogen = ?
Number of moles of hydrogen = 16.5 mol
Solution:
Formula:
Mass = number of moles × molar mass
Molar mass of hydrogen = 1.008 g/mol
Now we will put the values in formula:
Mass = number of moles × molar mass
Mass = 16.5 × 1.008 g/mol
Mass = 16.632 g
Calculate following in 5.6g of nitrogena) number of moles of N2b)number of molecules of N2c)number of atoms of nitrogen
Calculate following in 5.6g of nitrogena) number of moles of N2b)number of molecules of N2c)number of atoms of nitrogen
Given that 5.6g of nitrogen Atomic mass of nitrogen = 14u We know that, Molar atomic mass of nitrogen = 14g mol-1 Molar mass of N 2 = 2 x 14g mol-1 = 28g mol-1 a) No.of moles of N 2 in 5.6g of nitrogen = 5.6g/28g mol-1 =0.2 mol b) No.of molecules of N 2 in 5.6g of nitrogen = 6.023 x 1023 x no.of moles of N 2 = 6.023 x 1023 x 0.2 = 1.2 x 1023 c) One molecule of N 2 has two N atoms No of atoms of nitrogen = 2 x no.of N 2 molecules = 2 x 1.2 x 1023 = 2.4 x 1023
So you have finished reading the how many atoms are in 5.6 moles of na topic article, if you find this article useful, please share it. Thank you very much. See more:

