You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how many moles of ammonium nitrate are in 335 ml on Google, you do not find the information you need! Here are the best content compiled and compiled by the Chewathai27.com team, along with other related topics such as: how many moles of ammonium nitrate are in 335 ml how many moles of solute are in 250 ml of 2.0 m cacl2, how many moles of cacl2 are in 250 ml, how many moles of solute is required to make 200.0 ml of a 0.125 m solution, how many moles of solute are in 96 ml of 4.9 m caco3, how many moles of solute is in 2.50 liters of 2.00 m h₂so₄, how many moles of solute are in 250ml of 2.0m cacl2? how many grams of cacl2 is this?, how many moles of cacl2 are in 0.250 l of a solution that is 1.50 m cacl2, how many moles of cucl2 are present in 100.0 ml of 0.250 m cucl2 solution
Contents
How many moles are in ammonium nitrate?
That much ammonium nitrate contains 0.1005 moles . When you are asked to convert either grams to moles, or moles to grams, always think molar mass. A compound’s molar mass represents the weight of 1 mole of that respective compound.
How many moles of solute are in 250 ml of2 0 m CaCl2?
1 Answer. Ernest Z. The solution contains 0.50 mol or 55 g of CaCl2 .
What is the molar mass of ammonium nitrate?
How do I calculate moles?
- The formula for the number of moles formula is expressed as.
- Given.
- Number of moles formula is.
- Number of moles = Mass of substance / Mass of one mole.
- Number of moles = 95 / 86.94.
How do you calculate ammonium nitrate?
The molar mass of ammonium nitrate= the sum of the molar mass of all its constituents in the correct proportion. = 80.043.
How many moles of solute are there in 250 mL of a 0.10 M CaCl2 solution?
How many grams and moles of solute are there in 250, mL of a 0.10M CaCl2 solution? mole 0.10 mole 0.250x L X = 0% 025 mole K 0.025mole 1 110.989 Cac₂ = Imole Cacl₂ (25.
How do you calculate moles from molarity?
- Find the molarity and volume of your solution.
- Make sure that the units for the volume are the same as for the volume part of the molarity (e.g., mL and mol/mL).
- Multiply the volume by the molarity. This is the number of moles present.
How many atoms does ammonium nitrate?
The chemical formula of Ammonium nitrate is NH4NO3. It consists of 2 nitrogen atoms, 3 oxygen atoms and 4 hydrogen atoms. The molecular formula of ammonium nitrate is N2H4O3.
How many particles are in a mole?
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro’s Number (6.0221421 x 1023).
How do you calculate volume in ml?
Once you know both the density and the mass, divide the mass by the density to find the volume. If you want to calculate volume in milliliters, measure the weight in grams.
How do you find moles from volume and molarity?
Compute the volume of a solution in liters, given the number of moles and molarity, by dividing the number of moles by the molarity in units of moles per liter. For example, a solution containing 6.0 moles and a having a molarity of 3.0 moles per liter has a volume of 2.0 moles per liter.
How do you calculate grams to moles?
Divide the mass of the substance in grams by its molecular weight. This will give you the number of moles of that substance that are in the specified mass. For 12 g of water, (25 g)/(18.015 g/mol) = 0.666 moles.
What is the mass of 6.02 x10 23 platinum atoms?
1 Answer. Well 6.0221×1023 platinum atoms have a mass of 195.08⋅g , so you tell us.
How much nitrogen is present in ammonium nitrate?
ammonium nitrate, (NH4NO3), a salt of ammonia and nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers.
How many moles of ammonium nitrate are in 335 mL of 0.425M NH_4NO_3? | Socratic
- Article author: socratic.org
- Reviews from users: 49475
Ratings
- Top rated: 4.7
- Lowest rated: 1
- Summary of article content: Articles about How many moles of ammonium nitrate are in 335 mL of 0.425M NH_4NO_3? | Socratic 0.142 moles. Explanation: You could get fancy and say that this solutions contains zero moles of ammonium nitrate, but that would not be the … …
- Most searched keywords: Whether you are looking for How many moles of ammonium nitrate are in 335 mL of 0.425M NH_4NO_3? | Socratic 0.142 moles. Explanation: You could get fancy and say that this solutions contains zero moles of ammonium nitrate, but that would not be the … “0.142 moles” You could get fancy and say that this solutions contains zero moles of ammonium nitrate, but that would not be the answer the problem is looking for. Here’s why that is. A solution’s molarity will tell you how many moles of solute you get per liter of solution. The problem with soluble ionic compounds is that they dissociate completely in aqueous solution to form cations and anions. In this case, ammonium nitrate exists in solution as ammonium cations, “NH”_4^(+), and nitrate anions, “NO”_3^(-) “NH”_4″NO”_text(3(aq]) -> “NH”_text(4(aq])^(+) + “NO”_text(3(aq])^(-) This means that your solution contains no ammonium nitrate, so technically the concentration of ammonium nitrate is zero. Simply put, molarity was initially used as a way to express the concentration of a particular chemical species present in solution. (For more on that, go here). However, it is now common to use molarity as a was to express the concentration of a solute regardless of the form in which it exists in solutions. In your case, you know that the solution has a molariy of “0.425 mol L”^(-1) and a total volume of “335 mL”. To find how many moles of ammonium nitrate you have in solution, use the equation color(blue)(|bar(ul(color(white)(a/a)c = n_”solute”/V_”solution”color(white)(a/a)|))) Do not forget to convert the volume to liters by using the conversion factor “1 L” = 10^3″mL” You will have c = n/V implies n = c * V n = “0.425 mol” color(red)(cancel(color(black)(“L”^(-1)))) * 335 * 10^(-3)color(red)(cancel(color(black)(“L”))) = color(green)(|bar(ul(color(white)(a/a)”0.142 moles”color(white)(a/a)|))) The answer is rounded to three sig figs.
- Table of Contents:

brainly.com
- Article author: brainly.com
- Reviews from users: 34324
Ratings
- Top rated: 3.5
- Lowest rated: 1
- Summary of article content: Articles about brainly.com Moles = ? Solution: First of all convert volume from mL into L. … Results: Hence, when 0.142 moles of Ammonium nitrate taken and is added with … …
- Most searched keywords: Whether you are looking for brainly.com Moles = ? Solution: First of all convert volume from mL into L. … Results: Hence, when 0.142 moles of Ammonium nitrate taken and is added with …
- Table of Contents:

Answers for Homework on Molarity due for Friday, January 11th
- Article author: studylib.net
- Reviews from users: 16010
Ratings
- Top rated: 3.4
- Lowest rated: 1
- Summary of article content: Articles about Answers for Homework on Molarity due for Friday, January 11th How many moles of ammonium nitrate are in 335 mL of 0.425 M NH 4NO3? … Calculate the molarity of a solution containing 400 g CuSO4 in 4.00 L of solution. …
- Most searched keywords: Whether you are looking for Answers for Homework on Molarity due for Friday, January 11th How many moles of ammonium nitrate are in 335 mL of 0.425 M NH 4NO3? … Calculate the molarity of a solution containing 400 g CuSO4 in 4.00 L of solution. Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politicsFree essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics
- Table of Contents:

How many moles of ammonium nitrate are present in “8.047 g” of this substance? | Socratic
- Article author: socratic.org
- Reviews from users: 13719
Ratings
- Top rated: 4.8
- Lowest rated: 1
- Summary of article content: Articles about How many moles of ammonium nitrate are present in “8.047 g” of this substance? | Socratic Updating …
- Most searched keywords: Whether you are looking for How many moles of ammonium nitrate are present in “8.047 g” of this substance? | Socratic Updating That much ammonium nitrate contains “0.1005 moles”. When you are asked to convert either grams to moles, or moles to grams, always think molar mass. A compound’s molar mass represents the weight of 1 mole of that respective compound. You can either look up the molar mass of ammonium nitrate, NH_4NO_3, or add the molar masses of the atoms that comprise it. Since ammonium nitrate’s molar mass represents the mass of 1 mole, you can use it to determine how many moles you correspond to that many grams “molar mass” = “m”/”n” => “n” = “m”/”molar mass” In your case, ammonium nitrate’s molar mass is 80.052 g/mol, which means that n = (“8.047″cancel(“g”))/(“80.052″cancel(“g”)/”mol”) = “0.1005 moles” NH_4NO_3
- Table of Contents:

How many moles of solute are in 250 mL of 2.0M CaCl_2? How many grams of CaCl_2 is this? | Socratic
- Article author: socratic.org
- Reviews from users: 39502
Ratings
- Top rated: 4.5
- Lowest rated: 1
- Summary of article content: Articles about How many moles of solute are in 250 mL of 2.0M CaCl_2? How many grams of CaCl_2 is this? | Socratic Updating …
- Most searched keywords: Whether you are looking for How many moles of solute are in 250 mL of 2.0M CaCl_2? How many grams of CaCl_2 is this? | Socratic Updating The solution contains 0.50 mol or 55 g of “CaCl”_2. Moles of “CaCl”_2 You know there are 2.0 mol of “CaCl”_2 in 1 L of solution. In 250 mL of solution there are “0.250” cancel(“L soln”) × “2.0 mol CaCl”_2/(1 cancel(“L soln”)) = “0.50 mol CaCl”_2 Mass of “CaCl”_2 The mass of 1 mol of “CaCl”_2 is “(40.08 + 2 × 35.45) g” = “(40.08 + 70.90) g” = “110.98 g”. So the mass of “0.50 mol of CaCl”_2 is “0.50” cancel(“mol CaCl”_2) × “110.98 g CaCl”_2/(1 cancel(“mol CaCl”_2)) = “55 g CaCl”_2
- Table of Contents:

Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry – YouTube
- Article author: www.youtube.com
- Reviews from users: 40823
Ratings
- Top rated: 4.0
- Lowest rated: 1
- Summary of article content: Articles about Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry – YouTube Updating …
- Most searched keywords: Whether you are looking for Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry – YouTube Updating This chemistry video tutorial focuses on molarity and dilution problems. It shows you how to convert between molarity, grams, moles, and liters. It’s very …molarity problems, molarity stoichiometry, grams, molarity and dilution problems, moles, dilution calculations, concentration, molarity, dilution, equation, stoichiometry, formula, practice problems, solution stoichiometry, problems, actual yield, theoretical yield, percent yield, liters, volume, mass, limiting and excess reactant, solute, limiting reactant, excess reactant, unit conversion, dimensional analysis, solvent, solution, metals, activity series, titrations, acid base, redox
- Table of Contents:
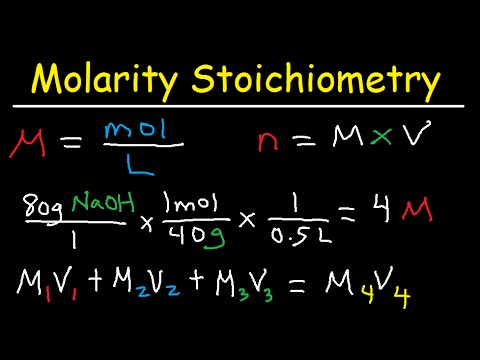
How many moles of ammonium nitrate are in..?
- Article author: teaching.education.narkive.com
- Reviews from users: 33109
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about How many moles of ammonium nitrate are in..? 335 mL of 0.425M NH4NO3(ammonium nitrate)?. Three answers: C S . 15 years ago. …
- Most searched keywords: Whether you are looking for How many moles of ammonium nitrate are in..? 335 mL of 0.425M NH4NO3(ammonium nitrate)?. Three answers: C S . 15 years ago.
- Table of Contents:

Error 403 (Forbidden)
- Article author: www.quora.com
- Reviews from users: 21966
Ratings
- Top rated: 4.6
- Lowest rated: 1
- Summary of article content: Articles about Error 403 (Forbidden) It tells us the number of moles (n) of solute per Litre of solution and is calculated … You need to calculate how many g are in 1 mol of ammonium nitrate. …
- Most searched keywords: Whether you are looking for Error 403 (Forbidden) It tells us the number of moles (n) of solute per Litre of solution and is calculated … You need to calculate how many g are in 1 mol of ammonium nitrate.
- Table of Contents:

how many moles of ammonium nitrate are in 335 ml
- Article author: wentzelchem.weebly.com
- Reviews from users: 10301
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about how many moles of ammonium nitrate are in 335 ml b) How many moles of ammonium nitrate are in 335 mL of 0.425 M NH4NO₂? … Calculate the molarity of a solution containing 400 g CuSO4. in 4.00 L of … …
- Most searched keywords: Whether you are looking for how many moles of ammonium nitrate are in 335 ml b) How many moles of ammonium nitrate are in 335 mL of 0.425 M NH4NO₂? … Calculate the molarity of a solution containing 400 g CuSO4. in 4.00 L of …
- Table of Contents:

Access to this page has been denied.
- Article author: www.chegg.com
- Reviews from users: 20519
Ratings
- Top rated: 4.3
- Lowest rated: 1
- Summary of article content: Articles about Access to this page has been denied. How many moles of ammonium nitrate are in 335mL of 0.425M NH.NO,? 16. How many moles of solute are in 250mL of 2.0M Caci,? How many grams of CaCl, is this? 17. …
- Most searched keywords: Whether you are looking for Access to this page has been denied. How many moles of ammonium nitrate are in 335mL of 0.425M NH.NO,? 16. How many moles of solute are in 250mL of 2.0M Caci,? How many grams of CaCl, is this? 17.
- Table of Contents:

See more articles in the same category here: Top 975 tips update new.
How many moles of ammonium nitrate are in 335 mL of 0.425M #NH_4NO_3#?
You could get fancy and say that this solutions contains zero moles of ammonium nitrate, but that would not be the answer the problem is looking for.
Here’s why that is.
A solution’s molarity will tell you how many moles of solute you get per liter of solution.
The problem with soluble ionic compounds is that they dissociate completely in aqueous solution to form cations and anions. In this case, ammonium nitrate exists in solution as ammonium cations, #”NH”_4^(+)# , and nitrate anions, #”NO”_3^(-)#
#”NH”_4″NO”_text(3(aq]) -> “NH”_text(4(aq])^(+) + “NO”_text(3(aq])^(-)#
This means that your solution contains no ammonium nitrate, so technically the concentration of ammonium nitrate is zero.
Simply put, molarity was initially used as a way to express the concentration of a particular chemical species present in solution. (For more on that, go here).
However, it is now common to use molarity as a was to express the concentration of a solute regardless of the form in which it exists in solutions.
In your case, you know that the solution has a molariy of #”0.425 mol L”^(-1)# and a total volume of #”335 mL”# . To find how many moles of ammonium nitrate you have in solution, use the equation
#color(blue)(|bar(ul(color(white)(a/a)c = n_”solute”/V_”solution”color(white)(a/a)|)))#
Do not forget to convert the volume to liters by using the conversion factor
#”1 L” = 10^3″mL”#
You will have
#c = n/V implies n = c * V# #n = “0.425 mol” color(red)(cancel(color(black)(“L”^(-1)))) * 335 * 10^(-3)color(red)(cancel(color(black)(“L”))) = color(green)(|bar(ul(color(white)(a/a)”0.142 moles”color(white)(a/a)|)))#
The answer is rounded to three sig figs.
How many moles of ammonium nitrate are present in #”8.047 g”# of this substance?
That much ammonium nitrate contains #”0.1005 moles”# .
When you are asked to convert either grams to moles, or moles to grams, always think molar mass.
A compound’s molar mass represents the weight of 1 mole of that respective compound. You can either look up the molar mass of ammonium nitrate, #NH_4NO_3# , or add the molar masses of the atoms that comprise it.
Since ammonium nitrate’s molar mass represents the mass of 1 mole, you can use it to determine how many moles you correspond to that many grams
#”molar mass” = “m”/”n” => “n” = “m”/”molar mass”#
In your case, ammonium nitrate’s molar mass is 80.052 g/mol, which means that
So you have finished reading the how many moles of ammonium nitrate are in 335 ml topic article, if you find this article useful, please share it. Thank you very much. See more: how many moles of solute are in 250 ml of 2.0 m cacl2, how many moles of cacl2 are in 250 ml, how many moles of solute is required to make 200.0 ml of a 0.125 m solution, how many moles of solute are in 96 ml of 4.9 m caco3, how many moles of solute is in 2.50 liters of 2.00 m h₂so₄, how many moles of solute are in 250ml of 2.0m cacl2? how many grams of cacl2 is this?, how many moles of cacl2 are in 0.250 l of a solution that is 1.50 m cacl2, how many moles of cucl2 are present in 100.0 ml of 0.250 m cucl2 solution

