You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how many valence electrons does fr have on Google, you do not find the information you need! Here are the best content compiled and compiled by the Chewathai27.com team, along with other related topics such as: how many valence electrons does fr have how many valence electrons does carbon have, how many valence electrons does ra have, how many valence electrons does beryllium have, how many valence electrons does he have, how many valence electrons does sulfur have, how many valence electrons does calcium have, how many valence electrons does si have, how many valence electrons does tellurium have?
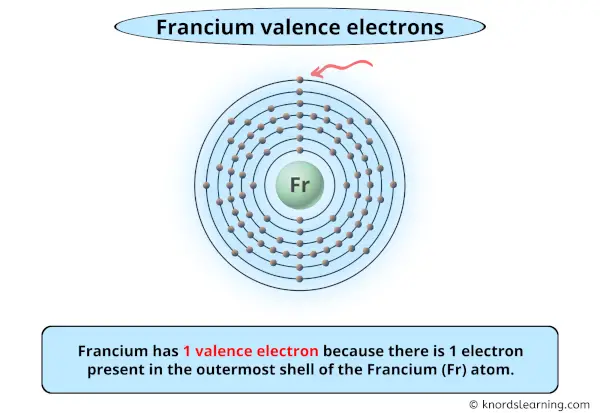
Francium has 1 valence electron because there is 1 electron present in the outermost shell of the Francium (Fr) atom.Fr+ is the ionized form of the element. The definition of the valence level is ‘the highest principle quantum number of the electronic configuration’. Since the 7s1 electron is no longer present in Fr[Rn]+ and n = 7 is the highest principle quantum number then there are no electrons in the valence level.Francium atom is an alkali metal atom. A radioactive alkali metal with the atomic symbol Fr, and atomic number 87. The mass numbers of known isotopes are 204-213, 217-224. Its valence is +1.
| Actinium | 3 | 4 |
|---|---|---|
| Fluorine | 1 | 3 |
| Francium | 1 | 3 |
| Gadolinium | 3 | 2 |
| Gallium | 3 | 4 |
| Atomic Number | 87 |
|---|---|
| Number of Electrons (with no charge) | 87 |
| Number of Protons | 87 |
| Mass Number | 223 |
| Number of Neutrons | 136 |
Contents
How many valence electrons does FR?
Fr+ is the ionized form of the element. The definition of the valence level is ‘the highest principle quantum number of the electronic configuration’. Since the 7s1 electron is no longer present in Fr[Rn]+ and n = 7 is the highest principle quantum number then there are no electrons in the valence level.
What is the Valency of FR?
| Actinium | 3 | 4 |
|---|---|---|
| Fluorine | 1 | 3 |
| Francium | 1 | 3 |
| Gadolinium | 3 | 2 |
| Gallium | 3 | 4 |
How many total electrons does FR have?
| Atomic Number | 87 |
|---|---|
| Number of Electrons (with no charge) | 87 |
| Number of Protons | 87 |
| Mass Number | 223 |
| Number of Neutrons | 136 |
What is the number of valence of francium?
Francium atom is an alkali metal atom. A radioactive alkali metal with the atomic symbol Fr, and atomic number 87. The mass numbers of known isotopes are 204-213, 217-224. Its valence is +1.
What group is fr?
francium (Fr), heaviest chemical element of Group 1 (Ia) in the periodic table, the alkali metal group. It exists only in short-lived radioactive forms. Natural francium cannot be isolated in visible, weighable amounts, for only 24.5 grams (0.86 ounce) occur at any time in the entire crust of Earth.
How do I find valence electrons?
For neutral atoms, the number of valence electrons is equal to the atom’s main group number. The main group number for an element can be found from its column on the periodic table. For example, carbon is in group 4 and has 4 valence electrons. Oxygen is in group 6 and has 6 valence electrons.
How many neutrons does FR have?
Francium is the heaviest of the naturally occuring alkali elements — the left most column of the periodic table of the elements. It has a 87 protons and between 120 to 140 neutrons. Its official symbol is Fr.
How many electrons does francium have in its outer shell?
All the Group 1 elements – lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr) – have one electron in the outer shell.
Why is francium named francium?
Word Origin: Francium was named for France, the country of its discovery. Discovery: Marguerite Perey discovered francium in 1939 at the Curie Institute in Paris. There are 33 recognized isotopes of francium.
How many neutrons does FR have?
Francium is the heaviest of the naturally occuring alkali elements — the left most column of the periodic table of the elements. It has a 87 protons and between 120 to 140 neutrons. Its official symbol is Fr.
How many valence electrons does copper atom have?
Yes, copper only has 1 valence electron.
How many electrons does francium have in its outer shell?
All the Group 1 elements – lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr) – have one electron in the outer shell.
How many valence Fe are there?
-From the electronic configuration of the iron atom, we come to know that the number of electrons present in the outermost shell i.e., 3 d orbital and 4 s orbital are 8 electrons. Thus, the total number of valence electrons present in iron is eight.
Francium Valence Electrons (And How to Find them?)
- Article author: knordslearning.com
- Reviews from users: 15712
Ratings
- Top rated: 3.3
- Lowest rated: 1
- Summary of article content: Articles about Francium Valence Electrons (And How to Find them?) Updating …
- Most searched keywords: Whether you are looking for Francium Valence Electrons (And How to Find them?) Updating So you have seen the above image by now, right?
- Table of Contents:
How to find the Valence Electrons (2 Methods)
Recent posts
How many valence electrons are in Fr^+? | Socratic
- Article author: socratic.org
- Reviews from users: 29972
Ratings
- Top rated: 4.8
- Lowest rated: 1
- Summary of article content: Articles about How many valence electrons are in Fr^+? | Socratic Updating …
- Most searched keywords: Whether you are looking for How many valence electrons are in Fr^+? | Socratic Updating zero Fr^+ is the ionized form of the element. The definition of the valence level is ‘the highest principle quantum number of the electronic configuration’. Fr^o[Rn]7s^1 + 1st Ionization Energy => Fr[Rn]^+ + 1e^- Since the 7s^1 electron is no longer present in Fr[Rn]^+ and n = 7 is the highest principle quantum number then there are no electrons in the valence level.
- Table of Contents:

Valence for all the elements in the Periodic Table
- Article author: periodictable.com
- Reviews from users: 46784
Ratings
- Top rated: 3.7
- Lowest rated: 1
- Summary of article content: Articles about Valence for all the elements in the Periodic Table Updating …
- Most searched keywords: Whether you are looking for Valence for all the elements in the Periodic Table Updating Complete and detailed technical data about the element $$$ELEMENTNAME$$$ in the Periodic Table.Periodic Table, Theodore Gray, Theo Gray, Chemical Elements, Elements, Chemistry
- Table of Contents:

Fr Francium Element Information: Facts, Properties, Trends, Uses and comparison – Periodic Table of the Elements | SchoolMyKids
- Article author: www.schoolmykids.com
- Reviews from users: 35541
Ratings
- Top rated: 4.0
- Lowest rated: 1
- Summary of article content: Articles about Fr Francium Element Information: Facts, Properties, Trends, Uses and comparison – Periodic Table of the Elements | SchoolMyKids Updating …
- Most searched keywords: Whether you are looking for Fr Francium Element Information: Facts, Properties, Trends, Uses and comparison – Periodic Table of the Elements | SchoolMyKids Updating Fr Francium Element information, facts. Francium properties, uses and trends | Periodic Table of the Elements – complete information about the francium element – Facts, atomic mass, melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron configuration, Chemical Properties francium, Isotopes, Health and Safety Parameters and Guidelines, Databases and comparison with other elements| SchoolMyKids Interactive Dynamic Periodic Table, Periodic Table Element Comparison tool, Element Property trendsFr Francium, electron configuration, Fr electron configuration, Francium electron configuration, Physical Properties, Chemical Properties of Francium, Shell Structure, Orbital Properties, Electronic Configuration, Francium Properties, Francium Melting Point, facts, Francium ion, Francium atomic mass, Francium boiling point, Francium uses
- Table of Contents:
Francium
Francium Facts
How to Locate Francium on Periodic Table
Table of Contents
Francium History
Francium Presence Abundance in Nature and Around Us
Crystal Structure of Francium
Francium Atomic and Orbital Properties
Francium Chemical Properties
Francium Ionization Energies and electron affinity
Francium Physical Properties
Francium Thermal Properties – Enthalpies and
thermodynamics
Francium Isotopes – Nuclear Properties of Francium
Regulatory and Health – Health and Safety Parameters and Guidelines
Database Search
Compare Francium with all Group 1 elements
Compare Francium with all alkali metal elements
Subscribe to our newsletter and get Parenting Schools & Health Updates Straight To Your Inbox

Francium | Fr – PubChem
- Article author: pubchem.ncbi.nlm.nih.gov
- Reviews from users: 24300
Ratings
- Top rated: 4.3
- Lowest rated: 1
- Summary of article content: Articles about Francium | Fr – PubChem Updating …
- Most searched keywords: Whether you are looking for Francium | Fr – PubChem Updating Francium | Fr | CID 6328145 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
- Table of Contents:
Valency of Francium | How many valence electrons does Francium (Fr) have?
- Article author: www.sciencecoverage.com
- Reviews from users: 10657
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about Valency of Francium | How many valence electrons does Francium (Fr) have? Valence electrons and valency of Fr+. Francium-ion Fr+ means it has lost one electron and has only 86 electrons in the orbitals. The electron … …
- Most searched keywords: Whether you are looking for Valency of Francium | How many valence electrons does Francium (Fr) have? Valence electrons and valency of Fr+. Francium-ion Fr+ means it has lost one electron and has only 86 electrons in the orbitals. The electron … Here you will know valency and valence electrons of Francium (Fr) in detail along with its electronic and molecular configuration.
- Table of Contents:

How Many Valence Electrons Does Francium Have?
- Article author: www.reference.com
- Reviews from users: 7962
Ratings
- Top rated: 4.0
- Lowest rated: 1
- Summary of article content: Articles about How Many Valence Electrons Does Francium Have? Francium has one valence electron as a member of the alkali metal group on the periodic table of elements. That valence electron is in the s-orbital of the … …
- Most searched keywords: Whether you are looking for How Many Valence Electrons Does Francium Have? Francium has one valence electron as a member of the alkali metal group on the periodic table of elements. That valence electron is in the s-orbital of the …
- Table of Contents:

How Many Valence Electrons Does Francium Have Archives – Dynamic Periodic Table of Elements and Chemistry
- Article author: periodictable.me
- Reviews from users: 23014
Ratings
- Top rated: 4.0
- Lowest rated: 1
- Summary of article content: Articles about How Many Valence Electrons Does Francium Have Archives – Dynamic Periodic Table of Elements and Chemistry Tags › How Many Valence Electrons Does Francium Have. October 11, 2018. Where To Find The Electron Configuration For Francium (Fr). no responses. …
- Most searched keywords: Whether you are looking for How Many Valence Electrons Does Francium Have Archives – Dynamic Periodic Table of Elements and Chemistry Tags › How Many Valence Electrons Does Francium Have. October 11, 2018. Where To Find The Electron Configuration For Francium (Fr). no responses.
- Table of Contents:
Where To Find The Electron Configuration For Francium (Fr)
Primary Sidebar

How many valence electrons are in Fr^+? | Socratic
- Article author: socratic.org
- Reviews from users: 27700
Ratings
- Top rated: 3.8
- Lowest rated: 1
- Summary of article content: Articles about How many valence electrons are in Fr^+? | Socratic Fr+ is the ionized form of the element. The definition of the valence level is ‘the highest principle quantum number of the electronic … …
- Most searched keywords: Whether you are looking for How many valence electrons are in Fr^+? | Socratic Fr+ is the ionized form of the element. The definition of the valence level is ‘the highest principle quantum number of the electronic … zero Fr^+ is the ionized form of the element. The definition of the valence level is ‘the highest principle quantum number of the electronic configuration’. Fr^o[Rn]7s^1 + 1st Ionization Energy => Fr[Rn]^+ + 1e^- Since the 7s^1 electron is no longer present in Fr[Rn]^+ and n = 7 is the highest principle quantum number then there are no electrons in the valence level.
- Table of Contents:

2022: ☢️ Valence Electrons in Francium (Fr) [& Facts, Color, Discovery …
- Article author: materials.gelsonluz.com
- Reviews from users: 49122
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about
2022: ☢️ Valence Electrons in Francium (Fr) [& Facts, Color, Discovery …
A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons … … - Most searched keywords: Whether you are looking for
2022: ☢️ Valence Electrons in Francium (Fr) [& Facts, Color, Discovery …
A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons … - Table of Contents:
Materials
Francium Overview
Video
Colored Periodic Table
Citation

How many valence electrons does francium have? – WorldsTerra
- Article author: worldsterra.com
- Reviews from users: 22385
Ratings
- Top rated: 3.9
- Lowest rated: 1
- Summary of article content: Articles about How many valence electrons does francium have? – WorldsTerra Francium has one valence electron as a member of the alkali metal group on the periodic table of elements. That valence electron is in the s-orbital of the … …
- Most searched keywords: Whether you are looking for How many valence electrons does francium have? – WorldsTerra Francium has one valence electron as a member of the alkali metal group on the periodic table of elements. That valence electron is in the s-orbital of the … Francium has one valence electron as a member of the alkali metal group on the periodic table of elements. That valence electron is in the s-orbital of the seventh energy level. The full electron configuration for francium is notated as 1s2 2s2p6 3s2p6d10 4s2p6d10f14 5s2p6d10 6s2p6 7s1, which can be simplified to [Rn] 7s1.
- Table of Contents:
How do you count valence electrons
What elements have 8 valence electrons
How do you calculate valence electrons
What period 4 element has 6 valence electrons

Valence for all the elements in the Periodic Table
- Article author: periodictable.com
- Reviews from users: 22642
Ratings
- Top rated: 4.5
- Lowest rated: 1
- Summary of article content: Articles about Valence for all the elements in the Periodic Table Actinium, 3, Hafnium, 4, Praseodymium, 4. Aluminum, 3, Hassium, N/A, Promethium, 3. Americium, 4, Helium, 0, Protactinium, 5. …
- Most searched keywords: Whether you are looking for Valence for all the elements in the Periodic Table Actinium, 3, Hafnium, 4, Praseodymium, 4. Aluminum, 3, Hassium, N/A, Promethium, 3. Americium, 4, Helium, 0, Protactinium, 5. Complete and detailed technical data about the element $$$ELEMENTNAME$$$ in the Periodic Table.Periodic Table, Theodore Gray, Theo Gray, Chemical Elements, Elements, Chemistry
- Table of Contents:

How many Valence Electrons does Group 14 have?
- Article author: byjus.com
- Reviews from users: 24525
Ratings
- Top rated: 4.4
- Lowest rated: 1
- Summary of article content: Articles about How many Valence Electrons does Group 14 have? How many Valence Electrons does Group 14 have? – The carbon family is Group 14. Carbon, silicon, germanium, tin, and lead are the five elements. …
- Most searched keywords: Whether you are looking for How many Valence Electrons does Group 14 have? How many Valence Electrons does Group 14 have? – The carbon family is Group 14. Carbon, silicon, germanium, tin, and lead are the five elements. How many Valence Electrons does Group 14 have? – The carbon family is Group 14. Carbon, silicon, germanium, tin, and lead are the five elements. There are four electrons in both of these elements at their outermost energy density. Visit BYJU’S to learn more about it.
- Table of Contents:

See more articles in the same category here: Top 975 tips update new.
Francium Valence Electrons (And How to Find them?)
So you have seen the above image by now, right?
Awesome! You can see that francium has 1 valence electron.
But how can you say that Francium has 1 valence electron
+
How can you find this valence electron?
Let’s discuss this in short.
Francium has 1 valence electron because there is 1 electron present in the outermost shell of the Francium (Fr) atom.
Now let’s see how you can easily find the valence electrons of Francium atom (Fr).
How to find the Valence Electrons? (2 Methods)
In order to find the valence electrons of Francium atom (Fr), you can use two methods.
Method 1: From the Periodic Table
To find out the valence electrons of Francium, you have to see the position of francium in the periodic table.
More specifically, you have to see the group wise position of Francium element in the periodic table.
From the above image, you can see that Francium (Fr) is present in the group 1 of periodic table.
(Note: Group 1 is also called group 1A).
So, as the francium element is present in group 1, it has 1 valence electron.
In this way, by knowing the position of francium element in periodic table, you can easily find its valence electrons.
Now let’s see another method for finding the number of valence electrons in francium.
Method 2: From the Electron Configuration
If you want to find the valence electrons of francium from its electron configuration, then you should know its electron configuration first.
Now there are many methods to write the electron configurations, but here I will show you the easiest method, i.e by using Aufbau principle.
Aufbau principle: The Aufbau principle simply states that the orbitals with the lower energy are filled first and then the orbitals with higher energy levels are filled.
According to the Aufbau principle, the orbitals are filled in the following order:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Also the maximum number of electrons that can be accommodated in s, p, d & f orbitals are mentioned in the below table.
Orbitals Maximum capacity of electrons s 2 p 6 d 10 f 14
Now let’s try to find the electron configuration of Francium by using the Aufbau principle.
Electron Configuration of Francium:
Follow the steps mentioned below to get the electron configuration of Francium.
To write the electron configuration of francium, we should first know the total number of electrons present in a francium atom.
The francium atom has a total of 87 electrons because its atomic number is 87 and it is a neutral atom.
Now we have to fill these 87 electrons in the atomic orbitals according to the Aufbau principle.
According to the Aufbau principle, the electrons will be filled first in 1s orbital, then in 2s orbital, then in 2p orbital, and so on…
So from the Aufbau principle, we can get the electron configuration of the francium atom as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 7s1.
Now in this electron configuration of francium, we have to see the total number of electrons present in the highest energy level.
You can see in the electron configuration of francium (1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 7s1) that the highest energy level is 7. And the total number of electrons present in this energy level is 1.
So by knowing the electron configuration, we have found that the Francium has 1 valence electron.
I hope you have understood the methods of finding the valence electrons in francium.
See more related topics for your practice;
Radium Valence Electrons
Boron Valence Electrons
Carbon Valence Electrons
Nitrogen Valence Electrons
Oxygen Valence Electrons
Fr Francium Element Information: Facts, Properties, Trends, Uses and comparison – Periodic Table of the Elements
Francium History
The element Francium was discovered by Marguerite Perey in year 1939 in France . Francium derived its name from Francia, the New Latin name for France
Francium Presence: Abundance in Nature and Around Us
The table below shows the abundance of Francium in Universe, Sun, Meteorites, Earth’s Crust, Oceans and Human Body.
Crystal Structure of Francium
The solid state structure of Francium is Body Centered Cubic.
The Crystal structure can be described in terms of its unit Cell. The unit Cells repeats itself in three dimensional space to form the structure.
Unit Cell Parameters
The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a, b and c)
a b c N/A
and the angles between them Lattice Angles (alpha, beta and gamma).
alpha beta gamma N/A
The positions of the atoms inside the unit cell are described by the set of atomic positions ( x i , y i , z i ) measured from a reference lattice point.
The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space are described by the 230 space groups (219 distinct types, or 230 if chiral copies are considered distinct.
Francium Atomic and Orbital Properties
Francium atoms have 87 electrons and the electronic shell structure is [2, 8, 18, 32, 18, 8, 1] with Atomic Term Symbol (Quantum Numbers) 2S 1/2 .
Atomic Number 87 Number of Electrons (with no charge) 87 Number of Protons 87 Mass Number 223 Number of Neutrons 136 Shell structure (Electrons per energy level) [2, 8, 18, 32, 18, 8, 1] Electron Configuration [Rn] 7s1 Valence Electrons 7s1 Oxidation State 1 Atomic Term Symbol (Quantum Numbers) 2S 1/2 Shell Structure of Francium – Electrons per energy level n s p d f 1 K 2 2 L 2 6 3 M 2 6 10 4 N 2 6 10 14 5 O 2 6 10 6 P 2 6 7 Q 1
Ground State Electronic Configuration of Francium – neutral Francium atom
The ground state electronic configuration of Neutral Francium atom is [Rn] 7s1. The portion of Francium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Rn]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.This is important as it is the Valence electrons 7s1, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Francium
Complete ground state electronic configuration for the Francium atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 7s1
Atomic Structure of Francium
Francium atomic radius is N/A, while it’s covalent radius is N/A.
Atomic Spectrum of Francium
Francium Chemical Properties: Francium Ionization Energies and electron affinity
The electron affinity of Francium is N/A
Valence 1 Electronegativity 0.7 ElectronAffinity N/A
Ionization Energy of Francium
Refer to table below for Ionization energies of Francium
Ionization energy number Enthalpy – kJ/mol 1 380
Francium Physical Properties
Refer to below table for Francium Physical Properties
Density N/A Molar Volume N/A cm3
Elastic Properties
Hardness of Francium – Tests to Measure of Hardness of Element
Francium Electrical Properties
Francium is N/A of electricity. Refer to table below for the Electrical properties of Francium
Francium Heat and Conduction Properties
Francium Magnetic Properties
Optical Properties of Francium
Acoustic Properties of Francium
Francium Thermal Properties – Enthalpies and thermodynamics
Refer to table below for Thermal properties of Francium
Enthalpies of Francium
Francium Isotopes – Nuclear Properties of Francium
Isotopes of rhodium. Naturally occurring Francium has 1 stable isotope – None.
Isotope Isotope Mass % Abundance T half Decay Mode 199Fr 200Fr 201Fr 202Fr 203Fr 204Fr 205Fr 206Fr 207Fr 208Fr 209Fr 210Fr 211Fr 212Fr 213Fr 214Fr 215Fr 216Fr 217Fr 218Fr 219Fr 220Fr 221Fr 222Fr 223Fr 224Fr 225Fr 226Fr 227Fr 228Fr 229Fr 230Fr 231Fr 232Fr
Regulatory and Health – Health and Safety Parameters and Guidelines
Database Search
List of unique identifiers to search the element in various chemical registry databases
WWW Error Blocked Diagnostic
Access Denied
Your access to the NCBI website at www.ncbi.nlm.nih.gov has been temporarily blocked due to a possible misuse/abuse situation involving your site. This is not an indication of a security issue such as a virus or attack. It could be something as simple as a run away script or learning how to better use E-utilities, http://www.ncbi.nlm.nih.gov/books/NBK25497/, for more efficient work such that your work does not impact the ability of other researchers to also use our site. To restore access and understand how to better interact with our site to avoid this in the future, please have your system administrator contact [email protected].
So you have finished reading the how many valence electrons does fr have topic article, if you find this article useful, please share it. Thank you very much. See more: how many valence electrons does carbon have, how many valence electrons does ra have, how many valence electrons does beryllium have, how many valence electrons does he have, how many valence electrons does sulfur have, how many valence electrons does calcium have, how many valence electrons does si have, how many valence electrons does tellurium have?

