You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how to make jwh-018 at home on Google, you do not find the information you need! Here are the best content compiled and compiled by the https://chewathai27.com team, along with other related topics such as: how to make jwh-018 at home how to make jwh at home, jwh-018 walmart, what is jwh-018, jwh-018 powder, jwh-018 long-term effects, jwh-018 alternative, ebay jwh-018 powder, jwh-250 spray
Contents
Where is JWH-018 found?
JWH 018 (Item No. 10900) is an analytical reference material categorized as a synthetic cannabinoid. It has been found in Spice/K2-type herbal blends and may have neurotoxic properties.
What is JWH-018 most commonly called?
Substance dependence
‘Spice’ refers to designer cannabinoids such as JWH-018, JWH-073, JWH-200 and (C8)-CP 47,497, initially found in ‘herbal smoking blends’; these are sold under many names, including K2, fake weed, Yucatan fire, skunk, moon rocks and others.
Who made JWH?
History. John W. Huffman, an organic chemist at Clemson University, synthesized a variety of chemical compounds that affect the endocannabinoid system. JWH-018 is one of these compounds, with studies showing an affinity for the cannabinoid (CB1) receptor five times greater than that of THC.
Is JWH-018 legal in the US?
Both substances are dangerous to an individual’s health, which is why both JWH-018 and marijuana are both listed as Schedule I Controlled Substances, which are banned from use.
What ingredients are in JWH-018?
JWH-018 (1-pentyl-3-(1-naphthoyl)indole or naphthalen-1-yl-(1-pentyl-1H-indol-3-yl)methanone) [Chemical Abstract Service (CAS) Registry Number 209414-07-3] has been identified as a substance that has some pharmacological similarities to the primary psychoactive constituent in marijuana (Cannabis sativa L.), Δ9-THC.
What is JWH made of?
JWH-018 is the 18th compound that Huffman’s research group synthesized in a series of more than 470 analogs and metabolites of ∆9-tetrahydrocannabinol (THC), the active component of marijuana. Huffman created these compounds to study their interactions with the cannabinoid receptors in the brain.
What is the strongest JWH?
The highest reported concentration of JWH-018 in our study was 87 ng/ml at 15 min after administration, which is about 9 to 17 times higher than the value published in a controlled human studies after inhalation18, 28, where doses are far away from toxic ones for humane and safety reasons.
What is the most powerful synthetic cannabinoid?
JWH-018, arguably the most widely known synthetic cannabinoid, belongs to the group of aminoalkylindoles and is considered to be three times as potent as THC. Aminoalkylindoles are by far the most prevalent compounds found in herbal products laced with synthetic cannabinoids.
What schedule drug is spice?
Be-‐ cause the chemicals used in Spice have a high potential for abuse and no medical benefit, the Drug Enforcement Admin-‐ istration (DEA) has designated the five active chemicals most frequently found in Spice as Schedule I controlled substances, making it illegal to sell, buy, or possess them.
What does JWH stand for?
| Acronym | Definition |
|---|---|
| JWH | John Wesley Harding |
| JWH | Journal of Women’s History |
| JWH | Julia Ward Howe (author) |
| JWH | John Wieland Homes (Smyrna, GA) |
What is Monkey Dust?
Otherwise known as Zombie Dust, Cannibal Dust, and methylenedioxypyrovalerone (MDPV), monkey dust is a synthetic cathinone or type of bath salt that is chemically similar to a naturally occurring stimulant called cathinone. Synthetic cathinones are part of a drug class called new psychoactive substances (NPS).
Is JWH 073 legal?
…
JWH-073.
| Legal status | |
|---|---|
| Legal status | CA : Schedule II DE : Anlage II (Authorized trade only, not prescriptible) UK : Class B US : Schedule I Illegal in Latvia & Poland |
Is JWH 250 legal?
While legal under federal law, products containing JWH-018 and JWH-073 are banned in several states and by the U. S. armed forces. At present, JWH-250 is illegal in only one state. Legal restrictions on these compunds are likely to be imposed nationwide.
What is the strongest synthetic cannabinoid?
JWH-018, arguably the most widely known synthetic cannabinoid, belongs to the group of aminoalkylindoles and is considered to be three times as potent as THC. Aminoalkylindoles are by far the most prevalent compounds found in herbal products laced with synthetic cannabinoids.
What does JWH stand for?
| Acronym | Definition |
|---|---|
| JWH | John Wesley Harding |
| JWH | Journal of Women’s History |
| JWH | Julia Ward Howe (author) |
| JWH | John Wieland Homes (Smyrna, GA) |
What is JWH-018 used for?
When administered to mice, JWH-018 produces effects consistent with other CB1 receptor agonists, including hypothermia, analgesia, reduced motor activity, and catalepsy (5, 6). In drug discrimination studies, JWH-018 fully substitutes for the THC stimulus cue in both mice and rats (7–9).
how to make jwh-018 at home
- Article author: www.quora.com
- Reviews from users: 42151
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about how to make jwh-018 at home They’re not chemically based on THC at all. AM2201 and JWH-018 are both designer drugs that have been found in Spice, K2, Kronic, Sage and other dangerous … …
- Most searched keywords: Whether you are looking for how to make jwh-018 at home They’re not chemically based on THC at all. AM2201 and JWH-018 are both designer drugs that have been found in Spice, K2, Kronic, Sage and other dangerous …
- Table of Contents:

JWH 018 (CAS 209414-07-3)
- Article author: www.caymanchem.com
- Reviews from users: 46905
Ratings
- Top rated: 3.0
- Lowest rated: 1
- Summary of article content: Articles about JWH 018 (CAS 209414-07-3) Updating …
- Most searched keywords: Whether you are looking for JWH 018 (CAS 209414-07-3) Updating Cayman Chemical Company supplies scientists worldwide with the resources necessary for advancing human and animal health. We manufacture high quality biochemicals, assay kits, antibodies, and recombinant proteins and offer contract services for custom chemical synthesis/analysis, assay development/screening, and drug discovery.
- Table of Contents:

ScienceDirect
- Article author: www.sciencedirect.com
- Reviews from users: 38462
Ratings
- Top rated: 3.5
- Lowest rated: 1
- Summary of article content: Articles about ScienceDirect Updating …
- Most searched keywords: Whether you are looking for ScienceDirect Updating
- Table of Contents:

JWH-018 – Wikipedia
- Article author: en.wikipedia.org
- Reviews from users: 36109
Ratings
- Top rated: 3.9
- Lowest rated: 1
- Summary of article content: Articles about JWH-018 – Wikipedia Updating …
- Most searched keywords: Whether you are looking for JWH-018 – Wikipedia Updating
- Table of Contents:
Contents
History[edit]
Pharmacology[edit]
Usage[edit]
Detection in biological fluids[edit]
Legal status[edit]
Synthesis[edit]
See also[edit]
References[edit]
External links[edit]
Navigation menu

5 Colorado residents indicted and arrested on drug charges, including conspiracy and possession with intent to manufacture and distribute | ICE
- Article author: www.ice.gov
- Reviews from users: 47089
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about 5 Colorado residents indicted and arrested on drug charges, including conspiracy and possession with intent to manufacture and distribute | ICE Updating …
- Most searched keywords: Whether you are looking for 5 Colorado residents indicted and arrested on drug charges, including conspiracy and possession with intent to manufacture and distribute | ICE Updating The five defendants arrested include: Dien Le, Ponlue Pim, Pirun Pim, Ricky Pim and Kenneth Barnes. They appeared in U.S. District Court in Denver July 22 where they were advised of their rights and the charges pending against them. They are due back in court July 25 for arraignment and a detention hearing.
- Table of Contents:
Main Navigation
Breadcrumb
Media Inquiries
Global Footer Navigation

Frontiers | Repeated Exposure to the “Spice” Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats
- Article author: www.frontiersin.org
- Reviews from users: 46558
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about Frontiers | Repeated Exposure to the “Spice” Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats Previous studies in mice have shown that repeated daily injections of THC or synthetic cannabinos produce behavioral tolerance due to … …
- Most searched keywords: Whether you are looking for Frontiers | Repeated Exposure to the “Spice” Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats Previous studies in mice have shown that repeated daily injections of THC or synthetic cannabinos produce behavioral tolerance due to … Naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) is a synthetic compound found in psychoactive “spice” products that activates cannabinoid receptors. Preclinical evidence suggests that exposure to synthetic cannabinoids increases 5-HT2A/2C receptor function in the brain, an effect which might contribute to psychotic symptoms. Here, we hypothesized that repeated exposures to JWH-018 would enhance behavioral responsiveness to the 5-HT2A/2C receptor agonist DOI. Male Sprague-Dawley rats fitted with subcutaneously (sc) temperature transponders received daily injections of JWH-018 (1.0 mg/kg, sc) or its vehicle for seven consecutive days. Body temperature and catalepsy scores were determined at 1, 2, and 4 h post-injection each day. At 1 and 7 days after the final repeated treatment, rats received a challenge injection of either DOI (0.1 mg/kg, sc) or the 5-HT1A receptor agonist 8-OH-DPAT (0.3 mg/kg, sc), then temperature and behavioral responses were assessed. Behaviors induced by DOI included wet dog shakes and back muscle contractions (i.e., skin jerks), while behaviors induced by 8-OH-DPAT included ambulation, forepaw treading, and flat body posture. On the first day of repeated treatment, JWH-018 produced robust hypothermia and catalepsy which lasted up to 4 h, and these effects were significantly blunted by day 7 of treatment. Repeated exposure to JWH-018 did not affect behaviors induced by DOI, but behavioral and hypothermic responses induced by 8-OH-DPAT were sig…Naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) is a synthetic compound found in psychoactive “spice” products that activates cannabinoid receptors. Preclinical evidence suggests that exposure to synthetic cannabinoids increases 5-HT2A/2C receptor function in the brain, an effect which might contribute to psychotic symptoms. Here, we hypothesized that repeated exposures to JWH-018 would enhance behavioral responsiveness to the 5-HT2A/2C receptor agonist DOI. Male Sprague-Dawley rats fitted with sc temperature transponders received daily injections of JWH-018 (1.0 mg/kg, sc) or its vehicle for 7 consecutive days. Body temperature and catalepsy scores were determined at 1, 2 and 4 h post-injection each day. At 1 and 7 days after the final repeated treatment, rats received a challenge injection of either DOI (0.1 mg/kg, sc) or the 5-HT1A receptor agonist 8-OH-DPAT (0.3 mg/kg, sc), then temperature and behavioral responses were assessed. Behaviors induced by DOI included wet dog shakes and back muscle contractions (i.e., skin jerks), while behaviors induced by 8-OH-DPAT included ambulation, forepaw treading and flat body posture. On the first day of repeated treatment, JWH-018 produced robust hypothermia and catalepsy which lasted up to 4 h, and these effects were significantly blunted by day 7 of treatment. Repeated exposure to JWH-018 did not affect behaviors induced by DOI, but behavioral and hypothermic responses induced by 8-OH-DPAT were significantly augmented 1 day after cessation of JWH-018 treatment. Collectively, our findings show that repeated treatment with JWH-018 produces tolerance to its hypothermic and cataleptic effects, which is accompanied by transient enhancement of 5-HT1A receptor sensitivity in vivo.5-HT2A receptor, 5-HT1A receptor, JWH-018, psychotic symptoms, Schizophrenia, Spice, synthetic cannabinoids
- Table of Contents:
ORIGINAL RESEARCH article
Introduction
Materials and Methods
Results
Discussion
Ethics Statement
Author Contributions
Conflict of Interest Statement
Funding
References
ScienceDirect
- Article author: www.sciencedirect.com
- Reviews from users: 14073
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about ScienceDirect Of all NPS entified, synthetic cannabinos make up an essential part (ie, 27–47% each year since 2009), totally counting to more than 100 during the period … …
- Most searched keywords: Whether you are looking for ScienceDirect Of all NPS entified, synthetic cannabinos make up an essential part (ie, 27–47% each year since 2009), totally counting to more than 100 during the period …
- Table of Contents:

JWH-018 – Wikipedia
- Article author: en.wikipedia.org
- Reviews from users: 11200
Ratings
- Top rated: 3.6
- Lowest rated: 1
- Summary of article content: Articles about JWH-018 – Wikipedia JWH-018 (1-pentyl-3-(1-naphthoyl)indole) or AM-678 is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and … …
- Most searched keywords: Whether you are looking for JWH-018 – Wikipedia JWH-018 (1-pentyl-3-(1-naphthoyl)indole) or AM-678 is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and …
- Table of Contents:
Contents
History[edit]
Pharmacology[edit]
Usage[edit]
Detection in biological fluids[edit]
Legal status[edit]
Synthesis[edit]
See also[edit]
References[edit]
External links[edit]
Navigation menu

Page not available – PMC
- Article author: www.ncbi.nlm.nih.gov
- Reviews from users: 33455
Ratings
- Top rated: 4.1
- Lowest rated: 1
- Summary of article content: Articles about Page not available – PMC Because we intentionally used higher doses which produce toxicity and induce seizures, these doses might have effects not representative of … …
- Most searched keywords: Whether you are looking for Page not available – PMC Because we intentionally used higher doses which produce toxicity and induce seizures, these doses might have effects not representative of …
- Table of Contents:

JWH-018 – YouTube
- Article author: www.youtube.com
- Reviews from users: 3858
Ratings
- Top rated: 4.6
- Lowest rated: 1
- Summary of article content: Articles about JWH-018 – YouTube JWH-018indole) or AM-678 is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 … …
- Most searched keywords: Whether you are looking for JWH-018 – YouTube JWH-018indole) or AM-678 is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 … JWH-018indole) or AM-678 is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, …video, chia sẻ, điện thoại có máy ảnh, điện thoại quay video, miễn phí, tải lên
- Table of Contents:

See more articles in the same category here: 670+ tips for you.
Wikipedia
Chemical compound
JWH-018 (1-pentyl-3-(1-naphthoyl)indole) or AM-678[1] is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB 1 and CB 2 cannabinoid receptors, with some selectivity for CB 2 . It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as “incense blends”.[2][3][4][5][6]
As a full agonist at both the CB 1 and CB 2 cannabinoid receptors, this chemical compound is classified as an analgesic medication.[7] The analgesic effects of cannabinoid ligands, mediated by CB1 receptors are well established in treatment of neuropathic pain, as well as cancer pain and arthritis.[7]
These compounds work by mimicking the body’s naturally-produced endocannabinoid hormones such as 2-AG and anandamide (AEA), which are biologically active and can exacerbate or inhibit nerve signaling.[7] As the cause is poorly understood in chronic pain states, more research and development must be done before the therapeutic potential of this class of biologic compounds can be realized.[7]
History [ edit ]
John W. Huffman, an organic chemist at Clemson University, synthesized a variety of chemical compounds that affect the endocannabinoid system. JWH-018 is one of these compounds, with studies showing an affinity for the cannabinoid (CB 1 ) receptor five times greater than that of THC. Cannabinoid receptors are found in mammalian brain and spleen tissue; however, the structural details of the active sites are currently unknown.[8]
On December 15, 2008, it was reported by German pharmaceutical companies that JWH-018 was found as one of the active components in at least three versions of the grey market drug Spice, which has been sold as an incense in a number of countries around the world since 2002.[9][10][11] An analysis of samples acquired four weeks after the German prohibition of JWH-018 took place found that the manufacturers had shortened the alkyl chain by one carbon to circumvent the ban.[12]
Pharmacology [ edit ]
JWH-018 is a full agonist of both the CB 1 and CB 2 cannabinoid receptors, with a reported binding affinity of 9.00 ± 5.00 nM at CB 1 and 2.94 ± 2.65 nM at CB 2 .[3] JWH-018 has an EC 50 of 102 nM for human CB 1 receptors, and 133 nM for human CB 2 receptors.[13] JWH-018 produces bradycardia and hypothermia in rats at doses of 0.3–3 mg/kg, suggesting potent cannabinoid-like activity.[13]
Pharmacokinetics [ edit ]
Metabolism of JWH-018 was assessed using Wistar rats which had been administered an ethanolic extract containing JWH-018. Urine was collected for 24 hours, followed by extraction of JWH-018 metabolites using both liquid-liquid extraction and solid-phase extraction. GC-MS was utilized to separate and identify the extracted compounds. JWH-018 and its N-dealkylated metabolite were only detected in small amounts, with hydroxylated N-dealkylated metabolites comprising the primary signal. The observed mass shift indicates that it is likely that hydroxylation occurs in both the naphthalene and indole portions of the molecule.[14] Human metabolites were similar although most metabolism took place on the indole ring and pentyl side chain, and the hydroxylated metabolites were extensively conjugated with glucuronide.[15]
Usage [ edit ]
At least one case of JWH-018 dependence has been reported by the media.[2] The user consumed JWH-018 daily for eight months. Withdrawal symptoms were more severe than those experienced as a result of cannabis dependence. JWH-018 has been shown to cause profound changes in CB 1 receptor density following administration, causing desensitization to its effects more rapidly than related cannabinoids.[6]
On October 15, 2011, Anderson County coroner Greg Shore attributed the death of a South Carolina college basketball player to “drug toxicity and organ failure” caused by JWH-018.[16] An email dated Nov 4, 2011 concerning the case was finally released by the city of Anderson S.C. on Dec 16, 2011 under the Freedom of Information Act after multiple requests by media to see the information had been denied.[17]
Compared to THC, which is a partial agonist at CB 1 receptors, JWH-018, and many synthetic cannabinoids, are full agonists. THC has been shown to inhibit GABA receptor neurotransmission in the brain via several pathways.[18][19] JWH-018 may cause intense anxiety, agitation, and, in rare cases (generally with non-regular JWH users), has been assumed to have been the cause of seizures and convulsions by inhibiting GABA neurotransmission more effectively than THC. Cannabinoid receptor full agonists may present serious dangers to the user when used to excess.[20]
Various physical and psychological adverse effects have been reported from JWH-018 use. One study reported psychotic relapses and anxiety symptoms in well-treated patients with mental illness following JWH-018 inhalation.[21] Due to concerns about the potential of JWH-018 and other synthetic cannabinoids to cause psychosis in vulnerable individuals, it has been recommended that people with risk factors for psychotic illnesses (like a past or family history of psychosis) not use these substances.[22]
Detection in biological fluids [ edit ]
JWH-018 usage is readily detected in urine using “spice” screening immunoassays from several manufacturers focused on both the parent drug and its omega-hydroxy and carboxyl metabolites.[23] JWH-018 will not be detected by older methods employed for detecting THC and other cannabis terpenoids. Determination of the parent drug in serum or its metabolites in urine has been accomplished by GC-MS or LC-MS. Serum JWH-018 concentrations are generally in the 1–10 μg/L range during the first few hours after recreational usage. The major urinary metabolite is a compound that is monohydroxylated on the omega minus one carbon atom of the alkyl side chain. A lesser metabolite monohydroxylated on the omega (terminal) position was present in the urine of 6 users of the drug at concentrations of 6–50 μg/L, primarily as a glucuronide conjugate.[24][25][26][27][28][29][30][31][32]
Legal status [ edit ]
JWH-018 powder as it was commonly sold online
Synthesis [ edit ]
See also [ edit ]
References [ edit ] [56]
5 Colorado residents indicted and arrested on drug charges, including conspiracy and possession with intent to manufacture and distribute
DENVER — Five Colorado residents were arrested late last week for conspiracy to distribute a controlled substance and possessing a controlled substance with intent to manufacture and distribute.
U.S. Attorney John Walsh, District of Colorado, announced the arrests. The controlled substance in this case is commonly known as Spice.
This investigation was conducted by U.S. Immigration and Customs Enforcement’s (ICE) Homeland Security Investigations (HSI). Playing a critical role in the investigation was the Northern Colorado Drug Task Force, which is made up of the Fort Collins Police Department, the Loveland Police Department, and Colorado Adult Parole.
The court unsealed the indictment Monday.
The five defendants arrested include: Dien Le, Ponlue Pim, Pirun Pim, Ricky Pim and Kenneth Barnes. They appeared in U.S. District Court in Denver July 22 where they were advised of their rights and the charges pending against them. They are due back in court July 25 for arraignment and a detention hearing.
On July 19, HSI special agents and Northern Colorado Task Force officers executed search warrants at seven locations, including residences and businesses in Fort Collins. During the course of executing those warrants, special agents and officers seized the following items: money from several bank accounts used by the defendants (amounts to be determined), $26,000 in cash, 75 pounds of Spice and the chemicals and dry products to make Spice, thousands of packaging units of Spice for later sale, and several firearms.
According to the indictment, from Oct. 11, 2012 through about April 30, 2013, and continuing within the State of Colorado and elsewhere, the defendants knowingly and intentionally conspired to manufacture, possess with the intent to distribute and to distribute mixtures or substances containing detectable amounts of JWH-018 [1-pentyl-3-(1-naphthoyl)indole], also known as synthetic cannabinoid, a Schedule I controlled substance. The street name for this drug is Spice.
The investigation revealed that Barnes ordered JWH-018, which is a white powder, from China. He had the powder delivered from China to New York City. From there, the illegal substance was sent from New York to Fort Collins, Colo. Barnes also had a green leafy substance sent to Fort Collins from San Antonio, Texas. In Fort Collins, Barnes, Le and the Pims mixed the substances together, wet it, and let it dry, thus creating Spice. They then packaged the product and took it to head shops, gas stations and other local stores to sell it. The cost of 1.5 gram packets was $10, and the cost for 3 gram packets was $20.
The product used by the defendants, JWH-018, is manufactured in China, with no Food and Drug Administration or other type of oversight. It can contain substances that are dangerous to an individuals’ health. Some purchase Spice because they cannot purchase marijuana. Both substances are dangerous to an individual’s health, which is why both JWH-018 and marijuana are both listed as Schedule I Controlled Substances, which are banned from use.
“Spice is a very dangerous substance that is being used by people as young as teenagers,” said U.S. Attorney John Walsh. “When a person uses Spice they have no actual idea what they are putting into their body — as a key part of the product is made in China without regulatory controls.”
“Illicit smuggling schemes involving synthetic marijuana pose a growing threat to public health and safety,” said Kumar Kibble, special agent in charge of HSI Denver. “Because these drugs are unregulated and untested, it is impossible to know what chemicals are being ingested, making them incredibly dangerous. With these latest arrests, HSI and our law enforcement partners have struck a huge blow to the synthetic drug industry.”
“This is another excellent example of federal and local law enforcement personnel working well together,” said Lt. Greg Yeager, commander of the Northern Colorado Drug Task Force. “The dismantling of this drug trafficking organization will have a lasting impact on the presence of illegal drugs not only in the city of Fort Collins, but across the nation.”
Those charged with conspiracy to distribute a controlled substance face not more than 20 years in federal prison, and a fine of up to $1 million. Those charged with possession of a controlled substance with intent to manufacture or distribute also face not more than 20 years in federal prison, and up to a $1 million fine, per count.
This case is being prosecuted by Assistant U.S. Attorney Jeremy Sibert, District of Colorado.
The charges contained in the indictment are allegations, and the defendants are presumed innocent unless and until proven guilty.
Repeated Exposure to the “Spice” Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats
Naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) is a synthetic compound found in psychoactive “spice” products that activates cannabinoid receptors. Preclinical evidence suggests that exposure to synthetic cannabinoids increases 5-HT 2A/2C receptor function in the brain, an effect which might contribute to psychotic symptoms. Here, we hypothesized that repeated exposures to JWH-018 would enhance behavioral responsiveness to the 5-HT 2A/2C receptor agonist DOI. Male Sprague-Dawley rats fitted with subcutaneously (sc) temperature transponders received daily injections of JWH-018 (1.0 mg/kg, sc) or its vehicle for seven consecutive days. Body temperature and catalepsy scores were determined at 1, 2, and 4 h post-injection each day. At 1 and 7 days after the final repeated treatment, rats received a challenge injection of either DOI (0.1 mg/kg, sc) or the 5-HT 1A receptor agonist 8-OH-DPAT (0.3 mg/kg, sc), then temperature and behavioral responses were assessed. Behaviors induced by DOI included wet dog shakes and back muscle contractions (i.e., skin jerks), while behaviors induced by 8-OH-DPAT included ambulation, forepaw treading, and flat body posture. On the first day of repeated treatment, JWH-018 produced robust hypothermia and catalepsy which lasted up to 4 h, and these effects were significantly blunted by day 7 of treatment. Repeated exposure to JWH-018 did not affect behaviors induced by DOI, but behavioral and hypothermic responses induced by 8-OH-DPAT were significantly augmented 1 day after cessation of JWH-018 treatment. Collectively, our findings show that repeated treatment with JWH-018 produces tolerance to its hypothermic and cataleptic effects, which is accompanied by transient enhancement of 5-HT 1A receptor sensitivity in vivo .
Introduction
Synthetic cannabinoids are novel psychoactive substances with pharmacological similarity to the phytocannabinoid Δ9-tetrahydrocannabinol (THC), the main psychoactive ingredient in marijuana. Over the last decade, herbal smoking blends consisting of plant material laced with synthetic cannabinoids (i.e., “spice” products) have emerged in the recreational drug marketplace. Analytical investigations of the first spice products revealed that a primary psychoactive component was naphthalen-1-yl-(1-pentylindol-3-yl)methanone, also known as JWH-018 (1, 2). JWH-018 and many of its structural analogs were found in spice products during 2010 through 2013, and JWH-018 is still present on the street today (3, 4). JWH-018 is a potent agonist at cannabinoid type-1 (CB 1 ) and cannabinoid type-2 (CB 2 ) receptors, which displays at least threefold higher binding affinity than THC at both receptors (5, 6). When administered to mice, JWH-018 produces effects consistent with other CB 1 receptor agonists, including hypothermia, analgesia, reduced motor activity, and catalepsy (5, 6). In drug discrimination studies, JWH-018 fully substitutes for the THC stimulus cue in both mice and rats (7–9).
Several lines of clinical evidence support a relationship between heavy cannabis use and risk for development of psychosis and schizophrenia (10–13). Although the precise underpinnings of schizophrenia are not fully understood, dysregulation of brain serotonin (5-HT) systems has been implicated in certain psychotic symptoms, such as paranoia and hallucinations (14–16). Preclinical studies in rodents show that exposure to CB 1 receptor agonists can influence 5-HT receptor responsiveness in vivo. For example, Darmani reported that acute pretreatment with various cannabinoids, including THC and the potent cannabinoid receptor agonist (6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6H,6aH,7H,10H,10aH-benzo[c]isochromen-1-ol (HU-210), inhibits behavioral effects of the 5-HT 2A/2C agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) in mice (17). By contrast, Hill et al. found that repeated treatment with HU-210 for 12 days enhances wet dog shakes induced by DOI in rats (18). Franklin et al. found that 7-day exposure to the cannabinoid agonist 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol (CP 55,940) enhances cortisoterone release induced by DOI in rats, and this effect is accompanied by upregulation of 5-HT 2A receptors in the hypothalamus (19). Recent evidence suggests a direct interaction between CB 1 and 5-HT 2A receptors in rat brain. In particular, Viñals et al. showed that CB 1 and 5-HT 2A receptor heteromers are present in hippocampus and other brain regions related to memory formation, and these heteromers are necessary for the amnesic effects of THC, but not its analgesic effects (20).
Despite the continued misuse of synthetic cannabinoids by humans, little is known about the functional consequences of repeated administration of JWH-018 or related substances found in spice products. Given the emerging evidence for interactions between cannabinoid and 5-HT systems in the brain, we sought to determine the effects of repeated treatment with JWH-018 on the behavioral responsiveness to selective 5-HT receptor agonists. Specifically, male Sprague-Dawley rats were treated for seven consecutive days with JWH-018, then challenged with DOI or the 5-HT 1A receptor agonist 8-hydroxy-2-(dipropylamino)tetralin (8-OH-DPAT) at 1 day and 7 days after the last JWH-018 treatment. Body temperatures and catalepsy scores were determined during the repeated dosing regimen of JWH-018, while body temperatures and agonist-induced behaviors were measured following challenge doses of 5-HT drugs. We hypothesized that repeated exposure to JWH-018 would enhance subsequent behavioral responsiveness to DOI in rats [e.g., see Ref. (18)]. Such increases in 5-HT 2A/2C activity produced by cannabinoid exposure could contribute to adverse psychiatric symptoms associated with cannabinoid use.
Materials and Methods
Drugs and Reagents
Naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) was obtained from Cayman Chemical (Ann Arbor, MI, USA). (−)-2,5-Dimethoxy-4-iodoamphetamine HCl (DOI) and (+)-8- hydroxy-2-(dipropylamino)tetralin HBr (8-OH-DPAT) were obtained from Sigma Aldrich (St. Louis, MO, USA). 5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide HCl (rimonabant) was obtained from the pharmacy at the National Institute on Drug Abuse (NIDA), Intramural Research Program (IRP). JWH-018 and rimonabant were dissolved into a 1:1:18 mix of dimethyl sulfoxide:Tween 80:sterile saline, whereas other drugs were dissolved in sterile saline. All injections were administered at a volume of 1.0 mL/kg.
Animals and Surgery
Male Sprague-Dawley rats (Envigo, Frederick, MD, USA) weighing 250–300 g were double-housed (lights on: 7:00 a.m.–7:00 p.m.) under conditions of controlled temperature (22 ± 2°C) and humidity (45 ± 5%) with free access to food and water. Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Vivarium facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and study procedures were approved by the NIDA IRP Animal Care and Use Committee. After 2 weeks of acclimation to the vivarium, rats were subjected to surgical procedures and subsequently used for experiments. Rats were rapidly anesthetized with isoflurane using a drop jar which contained a raised floor above a gauze pad saturated with 5 mL of isoflurane. Once fully anesthetized, each rat received a surgically implanted IPTT-300 transponder (Bio Medic Data Systems, Seaford, DE, USA) to facilitate the non-invasive measurement of body temperature via a portable radio frequency reader system (handheld reader). The transponders were 14 mm × 2 mm cylinders implanted subcutaneously (sc) posterior to the shoulder blades via a sterile guide needle. Animals were individually housed postoperatively and allowed 7–10 days for recovery.
Acute JWH-018 Administration and Rimonabant Antagonism
As a first step in our study, we examined the dose–response effects of acute JWH-018 administration in a cohort of 12 rats. Rats were tested once per week for three consecutive weeks. On test day, rats were moved to the testing room in their home cages and given 1 h to acclimate. Feeding trays were removed, and wire lids were placed atop the cages. Rats received sc injections of JWH-018 (0.1, 0.3, or 1.0 mg/kg) or its vehicle. Immediately before injection, and at various times thereafter (0.25, 0.5, 0.75, 1, 1.5, 2, and 4 h post-injection), body temperature was measured using the handheld reader, and animals were observed for 90 s to assess behaviors. Observers were not blind to the drug treatment condition. Rats were assigned a catalepsy score based on three behaviors: immobility (absence of movement), flattened body posture, and splayed limbs (limbs spread out away from the center of the body). Each behavior was given a numerical score of 1 for “behavior absent,” 2 for “behavior present,” or 3 for “behavior continuous/intense”; the three scores were summed to provide a single value ranging from 3 to 9 at each time point.
Once dose–response experiments were completed, we next tested the effect of pretreatment with the CB 1 receptor antagonist rimonabant on the responses induced by JWH-018 in a cohort of 12 rats. Rats were tested once per week for three consecutive weeks. Rats were pretreated with either 1.0 mg/kg of the CB 1 receptor antagonist rimonabant or its vehicle 30 min before injection with either 1.0 mg/kg JWH-018 or its vehicle. Body temperature measurements and behavior scoring were carried out as described previously for acute dose–response experiments.
Repeated Dosing with JWH-018
Results from the acute dose–response experiments demonstrated that 1.0 mg/kg JWH-018 produced robust hypothermia and catalepsy. Thus, this dose was used for the repeated injection experiments carried out in a group of 32 rats. The repeated dosing with JWH-018 or its vehicle was carried out in the vivarium. Rats fitted with surgically implanted sc temperature transponders received a single sc injection of either 1.0 mg/kg JWH-018 or its vehicle, and were returned to their home cages. Immediately before injection, and at 1, 2, and 4 h post-injection, body temperature was measured using the handheld reader, and animals were observed for 90 s. During the observation period, behaviors were scored using the catalepsy scale as detailed above in the Section “Acute JWH-018 Administration and Rimonabant Antagonism.” The JWH-018 injection procedure was repeated daily for seven consecutive days.
Challenge Injection with Serotonergic Agonists
One day after the last repeated treatment with JWH-018 or vehicle (i.e., day 8, or day 1 of withdrawal), rats were moved to the testing room in their home cages and given 1 h to acclimate. Feeding trays were removed, and wire lids were placed atop the cages. One cohort of 16 rats received 0.1 mg/kg of DOI, whereas another cohort of 16 rats received 0.3 mg/kg of 8-OH-DPAT. The doses of DOI and 8-OH-DPAT were based on preliminary dose–response experiments, which identified drug doses evoking robust behavioral changes that were less than maximal (data not shown). The specific non-contingent behaviors induced by DOI were wet dog shakes and back muscle contractions (i.e., skin jerks). Both behaviors are known to be mediated by 5-HT 2A receptors in rats (21–23). The numbers of wet dog shakes and skin jerks present during the observation period were tallied. Wet dog shakes were defined as a rapid and sudden rotation of the head, neck, and shoulders from one side to the other, analogous to the way a wet dog may shake to dry itself. Skin jerks were defined as brief paraspinal muscle contractions of the back muscles in a tail to head direction. Specific non-contingent behaviors induced by 8-OH-DPAT were locomotion in the horizontal plane (i.e., ambulation), forepaw treading, and flattened body posture, components of the 5-HT behavioral syndrome known to be mediated by 5-HT 1A receptors (24, 25). Possible scores for each behavior were 0 (behavior absent), 1 (behavior present), or 2 (behavior intense or continuous). At the end of the observation period, the scores for the three behaviors were summed to produce a 5-HT syndrome score for each time point.
After acute serotonergic drug challenge, body temperatures were measured using the handheld reader at 0.25, 0.5, 0.75, 1, 1.25, 1.5, and 2 h post-injection, and behavior scores were given at each time point as appropriate for the treatment received (i.e., wet dog shakes and skin jerks for DOI treatment, and serotonin syndrome scores for 8-OH-DPAT treatment). The acute challenge procedure with DOI and 8-OH-DPAT was repeated 1 week after the last repeated JWH-018 treatment.
Data Analysis and Statistics
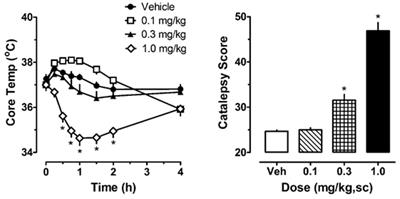
Data were tabulated, analyzed, and graphically depicted using GraphPad Prism (version 5.02; GraphPad Software, Inc., La Jolla, CA, USA). Time-course temperature data were analyzed using a two-way analysis of variance (treatment × time), followed by a Bonferroni post hoc test to determine significance between group means at specific time points. Mean temperature data from the DOI and 8-OH-DPAT experiments were evaluated by two-tailed t-tests. Catalepsy data from the acute dose–response, rimonabant antagonism and repeated treatments were analyzed by Kruskal–Wallis test (non-parametric), followed by Dunn’s multiple comparison test to determine significance between group means. Summed behavioral score data from the DOI and 8-OH-DPAT challenge experiments were analyzed using a Mann–Whitney test (non-parametric) comparing effects of repeated JWH-018 versus vehicle pretreatments. Statistical analyses were performed on data from all 7 days of the JWH-018 repeated administration experiment, however, Figure 3 only shows data from selected days to make the graphs easier to interpret. p < 0.05 was considered the minimal criterion for statistical significance. Results Effects of Acute JWH-018 Administration The left panel of Figure 1 illustrates the effect of acute JWH-018 administration on core body temperature in male rats. JWH-018 produced a dose-related change in core temperature (F 3,256 = 111.1, p < 0.0001), with significant reductions compared to vehicle control after the 1.0 mg/kg dose at 0.5, 0.75, 1, 1.5, and 2 h post-injection. A maximum decrease of ~3°C was observed at 1 h after the 1.0 mg/kg dose. It is worth noting that 0.1 mg/kg JWH-018 caused a noticeable, albeit non-significant, increase in temperature for the first 2 h, suggesting biphasic dose–response effects of the drug on body temperature. As seen in the right panel of Figure 1, JWH-018 dose-dependently increased the summed catalepsy behavioral score (Kruskal–Wallis statistic 28.53, p < 0.0001). Dunn’s test revealed that significant increases from vehicle control were present following the 1.0 mg/kg dose. FIGURE 1 Figure 1. Core temperature measures and summed catalepsy scores for rats receiving acute subcutaneous injections of 0.1, 0.3, and 1.0 mg/kg JWH-018 or its vehicle. Core temperature and behavioral score were recorded at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, and 4 h post-injection, as described in the Section “Acute JWH-018 Administration and Rimonabant Antagonism.” Data are mean ± SEM for N = 9 rats per group. *Represents significant effects when compared to the corresponding vehicle-treated group for temperature (Bonferroni, p < 0.05) and catalepsy (Dunn’s, p < 0.05). The left panel of Figure 2 shows that pretreatment with 1.0 mg/kg of the CB 1 receptor antagonist rimonabant significantly altered the hypothermic effect of 1.0 mg/kg JWH-018 (F 3,256 = 56.79, p < 0.0001). Rats treated with rimonabant/JWH-018 were not significantly different from rats treated with vehicle/vehicle, whereas the vehicle/JWH-018 group displayed decreased body temperature that was significantly different from all other groups. Vehicle/JWH-108 rats had significantly decreased body temperature at the 0.5, 0.75, 1, 1.5, and 2 h time points. Likewise, the right panel of Figure 2 shows that rats treated with vehicle/JWH-018 had significantly higher catalepsy scores when compared to all other groups (Kruskal–Wallis statistic 22.32, p < 0.0001). FIGURE 2 Figure 2. Core temperature measures and summed catalepsy scores for rats receiving either subcutaneous (sc) vehicle (VEH) or 1.0 mg/kg JWH-018 (JWH), 30 min after pretreatment with either sc vehicle (VEH) or 1.0 mg/kg rimonabant (RIM). Core temperature and behavioral score were recorded at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, and 4 h post-injection, as described in the Section “Acute JWH-018 Administration and Rimonabant Antagonism.” Data are mean ± SEM for N = 9 rats per group. *Represents significant effects when compared to the corresponding vehicle/vehicle-treated group for temperature (Bonferroni, p < 0.05) and catalepsy (Dunn’s, p < 0.05). Effects of Repeated JWH-018 Administration The left panel of Figure 3 depicts the effects of 1.0 mg/kg JWH-018 or its vehicle on body temperature on days 1, 3, 5, and 7 of repeated treatment. Vehicle administration did not significantly alter body temperature from preinjection values on day 1 of treatment, or during the 7-day treatment regimen (F 6,420 = 0.645, NS). Because vehicle administration did not affect body temperature over the course of repeated injections, we compared the effects of JWH-018 treatments across days to those of vehicle treatment on day 1. Using this analysis, JWH-018 caused significant hypothermia when compared to vehicle (F 7,480 = 22.331, p < 0.0001), but the temperature responses changed over the course of treatment. On day 1 of JWH-018 exposure, temperature was significantly reduced from vehicle at the 1, 2, and 4 h timepoints. By day 3 of treatment, hypothermia was observed only at the 1 h timepoint, and on days 6 and 7, no reduction in temperature was observed. FIGURE 3 Figure 3. Core temperature measures and summed catalepsy scores for rats receiving either subcutaneous vehicle (VEH) or 1.0 mg/kg JWH-018 (JWH) once daily for seven consecutive days. Core temperature and behavioral score were recorded at 0, 1, 2, and 4 h post-injection each day for 7 days, as described in the Section “Repeated dosing with JWH-018.” Data are mean ± SEM for N = 9 rats per group. *Represents significant effects when compared to the vehicle group from day 1 of treatment for temperature (Bonferroni, p < 0.05) and catalepsy (Dunn’s, p < 0.05). The right panel of Figure 3 depicts the effects of 1.0 mg/kg JWH-018 or its vehicle on summed catalepsy scores on days 1, 3, 5, and 7 of repeated treatment. Vehicle administration did not significantly alter summed catalepsy scores on day 1 of treatment, and there was no change in scores for vehicle-treated rats over the 7-day treatment regimen. Since vehicle administration did not change catalepsy scores over the course of treatment, we compared the effects of JWH-018 treatment across days to the effects of vehicle treatment on day 1. Using this analysis, JWH-018 increased catalepsy scores compared to vehicle (Kruskal–Wallis statistic 63.82, p < 0.0001), but the response was attenuated over the course of treatment. Dunn’s test demonstrated that summed catalepsy scores were significantly different when compared to vehicle on days 1 through 6, but not on day 7. Effects of Serotonergic Challenge with DOI and 8-OH-DPAT Figure 4 depicts the effects of the 5-HT 2A/2C receptor agonist DOI on wet dog shakes and skin jerks in rats given the daily regimen of 1.0 mg/kg JWH-018 or its vehicle for 7 days. Rats received 0.1 mg/kg DOI at 1 and 7 days after the last repeated JWH-018 injection. The data demonstrate that there were no significant differences between the pretreatment groups for induction of wet dog shakes (left panel) (Mann–Whitney = 29.50, p < 0.833) or skin jerks (right panel) (Mann–Whitney = 18.50, p < 0.171) at 1 day after cessation of JWH-018 administration. Similar non-significant effects between pretreatment groups were observed at day 7. DOI did not significantly affect core body temperature in rats pretreated with JWH-018 or vehicle at either test day (data not shown). FIGURE 4 Figure 4. Summed scores for wet dog shakes and back muscle crawls (skin jerks) induced by a subcutaneous challenge injection of 0.1 mg/kg DOI at 1 and 7 days after cessation of repeated JWH-018 treatment. Behavioral scores were recorded at 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, and 2 h post-injection, as described in the Section “Challenge injection with serotonergic agonists.” Data are mean ± SEM for N = 9 rats per group. A separate cohort of rats was given 0.3 mg/kg of the 5-HT 1A agonist 8-OH-DPAT at 1 and 7 days after the daily regimen of 1.0 mg/kg JWH-018 or its vehicle. Specific behaviors and hypothermic responses to 8-OH-DPAT were measured. The left panel of Figure 5 shows that there was a small yet significant enhancement of 5-HT syndrome score for the JWH-018 pretreated animals at 1 day after the last repeated treatment (Mann–Whitney = 9.50, p < 0.019), but this effect disappeared at 7 days (Mann–Whitney = 29.00, p < 0.791). The right panel of Figure 5 shows that mean hypothermic responses produced by 8-OH-DPAT were slightly lower in JWH-018 pretreated rats, but this effect was not significantly different between pretreatment groups day 1 (t = 1.854, df = 14, p < 0.085) or at day 7 (t = 1.925, df = 14, p < 0.075). FIGURE 5 Figure 5. Summed scores for serotonin syndrome behaviors and mean temperature recordings induced by a subcutaneous challenge injection of 0.3 mg/kg 8-OH-DPAT at 1 and 7 days after cessation of repeated JWH-018 treatment. Behavioral scores and core temperatures were recorded at 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, and 2 h post-injection, as described in the Section “Challenge injection with serotonergic agonists.” Data are mean ± SEM for N = 9 rats per group. *Represents significant effects when compared to the group that received repeated vehicle treatment (Mann–Whitney, p < 0.05). Because there were trends for enhanced hypothermic responses to 8-OH-DPAT in rats pretreated with JWH-018 (e.g., p < 0.07), we evaluated the raw time-course data for temperature responses in this experiment. Figure 6 illustrates that rats exposed to JWH-018 displayed enhanced hypothermic responses to 8-OH-DPAT when compared to vehicle-pretreated rats at day 1 after cessation of repeated treatments (F 1,126 = 17.74, p < 0.001). Post hoc tests revealed that temperature was significantly decreased in the JWH-018 group compared to the vehicle group at 1.25, 1.5, 1.75, and 2 h time points after injection of 8-OH-DPAT. The enhanced responsiveness to 8-OH-DPAT in the JWH-018 group was still evident at 7 days after cessation of treatment (F 1,126 = 23.26 p < 0.001), though post hoc tests found no differences between pretreatment groups at any time point. FIGURE 6 Figure 6. Time-course of core body temperature changes induced by a subcutaneous challenge injection of 0.3 mg/kg 8-OH-DPAT at 1 and 7 days after cessation of repeated JWH-018 treatment. Temperatures were recorded at 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, and 2 h post-injection, as described in the Section “Challenge injection with serotonergic agonists.” Data are mean ± SEM for N = 9 rats per group. *Represents significant effects when compared to vehicle-pretreatment group at specific time points (Bonferroni, p < 0.05). Discussion The psychiatric literature supports a strong relationship between heavy cannabis use and risk for subsequent psychosis and schizophrenia (12). In addition, misuse of synthetic cannabinoids such as JWH-018 and its analogs is associated with induction of more severe psychotic symptoms when compared to the effects of marijuana (26, 27). Previous studies in rats demonstrate that exposure to synthetic cannabinoids can induce enhanced sensitivity to 5-HT 2A/2C receptor activation (18) and upregulation of 5-HT 2A/2C receptors in specific brain regions (28, 29). The aim of the present study was to use the popular synthetic cannabinoid JWH-018 to further explore the relationship between repeated cannabinoid exposure and serotonergic dysregulation. JWH-018 is a potent non-selective cannabinoid receptor agonist that was found in the first generation of spice products (1, 2). The present experiments yielded three primary findings. First, in contrast to the results of others [e.g., see Ref. (18)], we detected no significant difference in responsiveness to the 5-HT 2A/2C receptor agonist DOI between rats pretreated with synthetic cannabinoids compared to those pretreated with vehicle. Second, we found a modest and significant enhancement of sensitivity to behavioral and hypothermic effects induced by 8-OH-DPAT in rats exposed to repeated injections of JWH-018. Finally, our data show that rats receiving daily injections of JWH-018 develop profound tolerance to its hypothermic and cataleptic effects, such that these effects are nearly absent after 7 days of treatment. In our experiments, male rats were subjected to seven consecutive days of JWH-018 injections, then given a challenge dose of either the 5-HT 2A/2C agonist DOI or the 5-HT 1A agonist 8-OH-DPAT at 1 and 7 days after cessation of the repeated dosing regimen. Typical behavioral responses to DOI administration in rats are wet dog shakes (analogous to the head twitch response in mice) and back muscle contractions, also known as skin jerks (21–23). These responses are accepted as specific indicators of 5-HT 2A receptor activation since the effects are blocked by selective 5-HT 2A receptor antagonists. We found no significant difference in the number of wet dog shakes or skin jerks induced by DOI between the cannabinoid-treated and vehicle-treated groups at either time point. Our findings differ from those of Hill et al., who reported that 12 days of HU-210 administration in rats increases DOI-induced wet dog shakes but decreases skin jerks (18). It is noteworthy that we observed trends for augmented wet dog shakes and attenuated skin jerks in rats exposed to JWH-018, but these effects did not reach significance, perhaps due to variability in the behavioral data. We also administered a submaximal dose of 0.1 mg/kg DOI for our experiments, whereas Hill et al. administered a 10-fold higher dose. Hill et al. theorized that the differential effects of HU-210 on the two behaviors induced by DOI could be due to region-specific changes in 5-HT 2A receptors caused by the cannabinoids. This hypothesis was later supported by the work of Franklin et al., who found that 7-day administration of CP 55,940 increased DOI-induced prolactin release, while producing no change in brain levels of 5-HT 2A receptor mRNA (19). It is well known that HU-210 displays a much longer time course of action when compared to other synthetic cannabinoids, including JWH-018, and may bind pseudo-irreversibly to the CB 1 receptor. Hruba and McMahon found that rhesus monkeys trained to discriminate THC from vehicle continued to emit drug-appropriate responses for 48 h after administration of HU-210, while such responses to THC and CP 55,940 ceased after 5 h. The same study found that rimonabant treatment increased the ED 50 values of THC and CP 55,940 discrimination by 12.5 fold, while only causing a 3.8-fold increase for HU-210 (30). Thus, the discrepancies between our results and those of Hill et al. could be due to the use of different cannabinoid agonists for the repeated treatment regimen. Darmani administered a range of doses of THC, HU-210 and CP 55,940 to mice, followed by an injection of DOI 20 min later, and found that the cannabinoids dose-dependently reduce DOI-induced behaviors (17). Our study used a repeated cannabinoid administration paradigm followed by the administration of DOI after 1 and 7 days of withdrawal, so this may help to explain the differences between our results and those of Darmani. The present findings in rats show that administration of CB 1 agonists causes considerable catalepsy (see Figures 1–3), so it seems possible that suppression of motor activity caused by acute cannabinoids could influence subsequent behavioral effects of 5-HT 2A receptor agonists. We purposefully designed our experiments to examine the responsiveness to 5-HT agonists at 1 and 7 days after the acute effects of cannabinoid administration had subsided. We found a modest yet significant increase in the behavioral and hypothermic effects induced by 8-OH-DPAT in rats receiving repeated JWH-018 treatments when compared to those receiving repeated vehicle treatments. The augmented sensitivity to 8-OH-DPAT resolved by 7 days after cannabinoid exposure. In a previous study, Hill et al. found that repeated injections of HU-210 for 12 consecutive days reduce the hypothermic and corticosterone responses produced by 8-OH-DPAT in vehicle-treated animals (18). Both hypothermia and corticosterone release are presumably mediated by 5-HT 1A receptors in the brain (31), thus Hill et al. found that repeated administration of HU-210 decreases 5-HT 1A activity in response to agonism, whereas we found the exact opposite in rats exposed to JWH-018. It seems possible that discrepancies between our results and those of Hill et al. could be due to the use of different cannabinoid agonists, as noted above. On the other hand, Zavitsanou et al. demonstrated that repeated injections of HU-210 increase 5-HT 1A receptor density and mRNA levels in the hippocampus and amygdala of male rats (32), a finding consistent with the possibility of enhanced 5-HT 1A receptor responsiveness after cannabinoid exposure. Our data demonstrating an increase in 5-HT 1A receptor sensitivity after exposure to JWH-018 is a unique finding, and its relationship to the development of psychiatric symptoms following cannabinoid exposure warrants further study. Future research should determine whether 5-HT 1A upregulation occurs after repeated exposure to other synthetic cannabinoids. Importantly, and in contrast to existing findings using other cannabinoid compounds, our data show that repeated exposure to JWH-018 does not induce robust alterations in 5-HT 2A receptor responsiveness, but increases 5-HT 1A responsiveness. In addition to assessing changes in serotonergic activity after cannabinoid exposure, one of the secondary aims of our study was to examine pharmacological responses to repeated JWH-018 injections. Rats in our study had implantable temperature transponders to facilitate the non-invasive measurement of body temperature. JWH-018 was shown to dose-dependently cause hypothermia and catalepsy, both of which were reversed by rimonabant (see Figure 2). The present data showing acute decreases in body temperature after JWH-018 administration in rats are consistent with previous findings from our laboratory and others, which show dose-related hypothermic effects of JWH-018 as assessed by radiotelemetry or rectal probes to measure core temperatures (33–36). As the repeated injection procedure progressed in our study, rats began to develop tolerance to both the hypothermic and cataleptic effects produced by JWH-018. By day 5 of repeated treatments, the effects of JWH-018 became submaximal at all time points, and continued to decrease in the two remaining days. By day 7 of repeated treatments, the temperature and cataleptic effects JWH-018 were not significantly different from vehicle-treated animals. Previous studies in mice have shown that repeated daily injections of THC or synthetic cannabinoids produce behavioral tolerance due to downregulation and desensitization of CB 1 receptors (37). Likewise, acute JWH-018 is reported to induce downregulation of CB 1 receptors in cultured neurons by a mechanism involving rapid receptor internalization (38). The experiments of Tai et al. showed that mice develop tolerance to the hypothermic effects of JWH-018, but not the locomotor suppressing effects (39). The apparently contradictory findings between our results and those of Tai et al. may be due to species-specific differences between rats and mice. Tai et al. also showed that mice repeatedly exposed to THC develop a cross tolerance to the effects of JWH-018. The development of tolerance to cannabis is well documented, and the demonstration of tolerance to JWH-018 could have important clinical implications (40, 41). Dose escalation in human THC users is often observed as a means to overcome cannabis tolerance, but this phenomenon likely will not cause acute bodily harm. By contrast, dose escalation with JWH-018 or other potent synthetic cannabinoids could be more dangerous. Typical adverse effects arising from synthetic cannabinoid use are tachycardia, agitation, and nausea; more serious adverse events include seizures, acute kidney injury, new onset psychosis, severe cardiac crisis, and death (27, 42). Further research is required to determine if such dose escalation occurs in humans who use synthetic cannabinoids. To summarize, we found that repeated treatment with the synthetic cannabinoid JWH-018 does not lead to significant changes in 5-HT 2A receptor responsiveness in rats, but produces transient increases in 5-HT 1A receptor responsiveness. These findings, unlike data generated using other synthetic cannabinoids, do not support the contention that exposure to cannabinoid receptor agonists universally leads to an increase in 5-HT 2A receptor responsiveness, suggesting that alteration of 5-HT 2A neurotransmission may not be responsible for the link between cannabinoid exposure and the subsequent development of psychotic symptoms. On the other hand, rats in our experiments developed tolerance to both hypothermia and catalepsy produced by JWH-018 after several consecutive days of treatment, findings which differ from prior work in mice suggesting that tolerance only develops to hypothermic effects. Synthetic cannabinoid tolerance in humans could potentially lead to dose escalation, which could be more dangerous with synthetic cannabinoids when compared to marijuana. Ethics Statement Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Vivarium facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and study procedures were approved by the NIDA Intramural Research Program Animal Care and Use Committee. Author Contributions JE and MB were responsible for experiment design, statistical analysis, and manuscript writing. JE collected the data. Conflict of Interest Statement The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Funding This research was generously supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, DA-00523 (MB). References 1. Auwärter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom (2009) 44:832–7. doi:10.1002/jms.1558 CrossRef Full Text | Google Scholar 2. Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom (2010) 45:1186–94. doi:10.1002/jms.1811 PubMed Abstract | CrossRef Full Text | Google Scholar 3. U.S. Drug Enforcement Administration, Office of Diversion Control. National Forensic Laboratory Information System: Year 2013 Annual Report. Springfield, VA: U.S. Drug Enforcement Administration (2014). Google Scholar 4. U.S. Drug Enforcement Administration, Diversion Control Division. National Forensic Laboratory Information System: Year 2015 Annual Report. Springfield, VA: U.S. Drug Enforcement Administration (2016). Google Scholar 5. Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther (1998) 285:995–1004. PubMed Abstract | Google Scholar 6. Wiley JL, Marusich JA, Martin BR, Huffman JW. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend (2012) 123:148–53. doi:10.1016/j.drugalcdep.2011.11.001 PubMed Abstract | CrossRef Full Text | Google Scholar 7. Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE. Differential drug-drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: implications for drug abuse liability and pain therapy. J Pharmacol Exp Ther (2013) 346:350–61. doi:10.1124/jpet.113.206003 PubMed Abstract | CrossRef Full Text | Google Scholar 8. Gatch MB, Forster MJ. Delta9-tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav Pharmacol (2014) 25:750–7. doi:10.1097/FBP.0000000000000093 CrossRef Full Text | Google Scholar 9. Wiley JL, Lefever TW, Cortes RA, Marusich JA. Cross-substitution of delta9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav (2014) 124:123–8. doi:10.1016/j.pbb.2014.05.016 CrossRef Full Text | Google Scholar 10. Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull (2014) 40:1509–17. doi:10.1093/schbul/sbt181 PubMed Abstract | CrossRef Full Text | Google Scholar 11. Giordano GN, Ohlsson H, Sundquist K, Sundquist J, Kendler KS. The association between cannabis abuse and subsequent schizophrenia: a Swedish national co-relative control study. Psychol Med (2015) 45:407–14. doi:10.1017/S0033291714001524 PubMed Abstract | CrossRef Full Text | Google Scholar 13. Murray RM, Quigley H, Quattrone D, Englund A, Di Forti M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry (2016) 15:195–204. doi:10.1002/wps.20341 PubMed Abstract | CrossRef Full Text | Google Scholar 14. Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia. A postmortem study. Arch Gen Psychiatry (1993) 50:810–8. doi:10.1001/archpsyc.1993.01820220066007 PubMed Abstract | CrossRef Full Text | Google Scholar 15. Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry (2004) 55:225–33. doi:10.1016/j.biopsych.2003.09.017 PubMed Abstract | CrossRef Full Text | Google Scholar 16. Scarr E, Copolov DL, Dean B. A proposed pathological model in the hippocampus of subjects with schizophrenia. Clin Exp Pharmacol Physiol (2001) 28:70–3. doi:10.1046/j.1440-1681.2001.03400.x PubMed Abstract | CrossRef Full Text | Google Scholar 18. Hill MN, Sun JC, Tse MT, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int J Neuropsychopharmacol (2006) 9:277–86. doi:10.1017/S1461145705005651 PubMed Abstract | CrossRef Full Text | Google Scholar 19. Franklin JM, Mathew M, Carrasco GA. Cannabinoid-induced upregulation of serotonin 2A receptors in the hypothalamic paraventricular nucleus and anxiety-like behaviors in rats. Neurosci Lett (2013) 548:165–9. doi:10.1016/j.neulet.2013.05.039 PubMed Abstract | CrossRef Full Text | Google Scholar 20. Viñals X, Moreno E, Lanfumey L, Cordomí A, Pastor A, de La Torre R, et al. Cognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol (2015) 13:e1002194. doi:10.1371/journal.pbio.1002194 PubMed Abstract | CrossRef Full Text | Google Scholar 21. Fone KC, Johnson JV, Bennett GW, Marsden CA. Involvement of 5-HT2 receptors in the behaviours produced by intrathecal administration of selected 5-HT agonists and the TRH analogue (CG 3509) to rats. Br J Pharmacol (1989) 96:599–608. doi:10.1111/j.1476-5381.1989.tb11858.x PubMed Abstract | CrossRef Full Text | Google Scholar 22. Pranzatelli MR. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropane)-2. Neurosci Lett (1990) 115:74–80. doi:10.1016/0304-3940(90)90520-J CrossRef Full Text | Google Scholar 23. Wettstein JG, Host M, Hitchcock JM. Selectivity of action of typical and atypical anti-psychotic drugs as antagonists of the behavioral effects of 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane. Prog Neuropsychopharmacol Biol Psychiatry (1999) 23:533–44. doi:10.1016/S0278-5846(99)00014-7 CrossRef Full Text | Google Scholar 24. O’Connell MT, Curzon G. A comparison of the effects of 8-OH-DPAT pretreatment of different behavioural responses to 8-OH-DPAT. Eur J Pharmacol (1996) 312:137–43. doi:10.1016/0014-2999(96)00496-7 PubMed Abstract | CrossRef Full Text | Google Scholar 25. Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol (1984) 106:271–82. doi:10.1016/0014-2999(84)90714-3 PubMed Abstract | CrossRef Full Text | Google Scholar 26. Bassir Nia A, Medrano B, Perkel C, Galynker I, Hurd YL. Psychiatric comorbidity associated with synthetic cannabinoid use compared to cannabis. J Psychopharmacol (2016) 30:1321–30. doi:10.1177/0269881116658990 PubMed Abstract | CrossRef Full Text | Google Scholar 27. Fattore L. Synthetic cannabinoids-further evidence supporting the relationship between cannabinoids and ssychosis. Biol Psychiatry (2016) 79:539–48. doi:10.1016/j.biopsych.2016.02.001 CrossRef Full Text | Google Scholar 28. Franklin JM, Carrasco GA. Cannabinoid-induced enhanced interaction and protein levels of serotonin 5-HT(2A) and dopamine D(2) receptors in rat prefrontal cortex. J Psychopharmacol (2012) 26:1333–47. doi:10.1177/0269881112450786 CrossRef Full Text | Google Scholar 29. Franklin JM, Carrasco GA. G-protein receptor kinase 5 regulates the cannabinoid receptor 2-induced up-regulation of serotonin 2A receptors. J Biol Chem (2013) 288:15712–24. doi:10.1074/jbc.M113.454843 PubMed Abstract | CrossRef Full Text | Google Scholar 31. Larsson LG, Renyi L, Ross SB, Svensson B, Angeby-Moller K. Different effects on the responses of functional pre- and postsynaptic 5-HT1A receptors by repeated treatment of rats with the 5-HT1A receptor agonist 8-OH-DPAT. Neuropharmacology (1990) 29:85–91. doi:10.1016/0028-3908(90)90047-U PubMed Abstract | CrossRef Full Text | Google Scholar 32. Zavitsanou K, Wang H, Dalton VS, Nguyen V. Cannabinoid administration increases 5HT1A receptor binding and mRNA expression in the hippocampus of adult but not adolescent rats. Neuroscience (2010) 169:315–24. doi:10.1016/j.neuroscience.2010.04.005 PubMed Abstract | CrossRef Full Text | Google Scholar 33. Banister SD, Wilkinson SM, Longworth M, Stuart J, Apetz N, English K, et al. The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse. ACS Chem Neurosci (2013) 4:1081–92. doi:10.1021/cn400035r PubMed Abstract | CrossRef Full Text | Google Scholar 34. Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, et al. Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem Neurosci (2015) 6:1445–58. doi:10.1021/acschemneuro.5b00107 PubMed Abstract | CrossRef Full Text | Google Scholar 35. De Luca MA, Bimpisidis Z, Melis M, Marti M, Caboni P, Valentini V, et al. Stimulation of in vivo dopamine transmission and intravenous self-administration in rats and mice by JWH-018, a Spice cannabinoid. Neuropharmacology (2015) 99:705–14. doi:10.1016/j.neuropharm.2015.08.041 CrossRef Full Text | Google Scholar 36. Schindler CW, Gramling BR, Justinova Z, Thorndike EB, Baumann MH. Synthetic cannabinoids found in “spice” products alter body temperature and cardiovascular parameters in conscious male rats. Drug Alcohol Depend (2017) 179:387–94. doi:10.1016/j.drugalcdep.2017.07.029 CrossRef Full Text | Google Scholar 37. Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther (2002) 303:36–44. doi:10.1124/jpet.102.035618 CrossRef Full Text | Google Scholar 38. Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol (2010) 160:585–93. doi:10.1111/j.1476-5381.2009.00582.x PubMed Abstract | CrossRef Full Text | Google Scholar 39. Tai S, Hyatt WS, Gu C, Franks LN, Vasiljevik T, Brents LK, et al. Repeated administration of phytocannabinoid delta(9)-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol Res (2015) 102:22–32. doi:10.1016/j.phrs.2015.09.006 CrossRef Full Text | Google Scholar 41. Järbe TUC, Raghav JG. Tripping with synthetic cannabinoids (“spice”): anecdotal and experimental observations in animals and man. Curr Top Behav Neurosci (2017) 32:263–81. doi:10.1007/7854_2016_16 CrossRef Full Text | Google Scholar
So you have finished reading the how to make jwh-018 at home topic article, if you find this article useful, please share it. Thank you very much. See more: how to make jwh at home, jwh-018 walmart, what is jwh-018, jwh-018 powder, jwh-018 long-term effects, jwh-018 alternative, ebay jwh-018 powder, jwh-250 spray

