You are looking for information, articles, knowledge about the topic nail salons open on sunday near me how to melt titanium on Google, you do not find the information you need! Here are the best content compiled and compiled by the https://chewathai27.com team, along with other related topics such as: how to melt titanium how to melt titanium in terraria, titanium forging, titanium melting temp, how to make titanium, titanium investment casting, titanium breaking, titanium die casting, titanium axe
Titanium for serious applications is commonly melted in an induction furnace with inert gas or vacuum to prevent air contamination and actual combustion . For critical components the entire melting and casting process is done in vacuum or inert gas environment and with controlled temperatures at all points .As indicated by its negative redox potential, titanium is thermodynamically a very reactive metal that burns in normal atmosphere at lower temperatures than the melting point. Melting is possible only in an inert atmosphere or in a vacuum. At 550 °C (1,022 °F), it combines with chlorine.As in electroslag melting, the ingot was melted under a cover of molten slag, but melting was accomplished by induction heating. Titanium, because of its reactive nature, is melted almost exclusively by consumable electrode vacuum-arc melting.
Contents
Is titanium hard to melt?
As indicated by its negative redox potential, titanium is thermodynamically a very reactive metal that burns in normal atmosphere at lower temperatures than the melting point. Melting is possible only in an inert atmosphere or in a vacuum. At 550 °C (1,022 °F), it combines with chlorine.
Is there a way to melt titanium?
As in electroslag melting, the ingot was melted under a cover of molten slag, but melting was accomplished by induction heating. Titanium, because of its reactive nature, is melted almost exclusively by consumable electrode vacuum-arc melting.
How hot do you need to melt titanium?
Towards the high end of melting point extremes is tungsten (and titanium for more commonly used metals). Tungsten has the highest melting point coming in at the extremely high temperature of 6,150 °F / 3,399 °C, while titanium melts at 3,040 °F / 1,670 °C.
What furnace can melt titanium?
Although this technology has evolved considerably over the past 30 years, the VAR furnace is still the workhorse for producing titanium ingots and castings.
Is titanium toxic to humans?
Safe in the body
Titanium is considered the most biocompatible metal – not harmful or toxic to living tissue – due to its resistance to corrosion from bodily fluids. This ability to withstand the harsh bodily environment is a result of the protective oxide film that forms naturally in the presence of oxygen.
What can melt titanium?
Currently, almost all titanium alloys are melted and cast by vacuum arc remelting (VAR) or induction skull melting using rammed graphite moulds for casting. A typical example of this is the use of a cold crucible for skull melting [25].
What is titanium worth?
| Year | Price | Price (Inflation Adjusted) |
|---|---|---|
| 2018 | $4,800.00 | $4,800.00 |
| 2017 | $4,150.00 | $4,249.60 |
| 2016 | $4,100.00 | $4,294.96 |
| 2015 | $5,200.00 | $5,572.56 |
Can titanium withstand a bullet?
Titanium, however doesn’t stand a chance against bullets fired from high-powered military grade firearms such as those used to penetrate tanks. Titanium can take single hits from high-caliber bullets, but it shatters and becomes penetrable with multiple hits from military-grade, armor piercing bullets.
Can titanium be broken?
Titanium metal is brittle when cold and can break apart easily at room temperature. At higher temperatures, it becomes malleable and ductile. Malleable means capable of being hammered into thin sheets.
Can you boil titanium?
Titanium pots are ideal primarily for boiling water because they can be made with thin walls, and transfer heat very quickly. Like stainless steel pots, they tend to develop hot spots, making them less than ideal for cooking real meals.
Does titanium melt in fire?
According to the reference [24], the flame temperature of titanium alloy is about 2930 °C which is above its melting point. Therefore, the titanium alloy begins to melt into liquid phase during combustion process.
What is titanium flammable?
Titanium powder is FLAMMABLE and SPONTANEOUSLY COMBUSTIBLE. Use dry chemical, sand or lime as extinguishing agents. DO NOT USE WATER on MOLTEN or BURNING TITANIUM as an explosion may occur. POISONOUS GASES ARE PRODUCED IN FIRE, including Titanium Oxides. CONTAINERS MAY EXPLODE IN FIRE.
Can titanium be induction heated?
Induction heating has important applications in science and industry. The method of induction heating can be successfully used for melting and heat treatment of titanium and zirconium alloys.
Can you melt and cast titanium?
The difficulties are that titanium must be handled more carefully because molten titanium is very reactive to liquids, gases, and solids. At present, consumable vacuum arc melting offers the only suitable commercial method of producing titanium castings.
What is the best material for a crucible?
Alumina (Al2O3) Crucibles
It is the most commonly used crucible for lab research use. The material has a high melting point is and relatively chemically inert.
Is titanium hard or tough?
Titanium is a natural metal that is frequently referred to by media and filmmakers as an extra-hard material. Its strength-weight ratio is almost double that of steel alloys.
Why is titanium difficult to machine?
The likely reasons for poor machinability of titanium alloys are poor thermal conductivity, low modulus of elasticity, dynamic shear strength, high chemical reactivity, and high hot hardness.
Is titanium considered a hard metal?
Even in its pure form, titanium is harder than many steel forms. As a refractory metal, it is highly resistant to heat and abrasion which is why titanium and its alloys are popular . It can be alloyed with iron and carbon, for example.
Can titanium withstand a bullet?
Titanium, however doesn’t stand a chance against bullets fired from high-powered military grade firearms such as those used to penetrate tanks. Titanium can take single hits from high-caliber bullets, but it shatters and becomes penetrable with multiple hits from military-grade, armor piercing bullets.
How could I melt Titanium? | Physics Forums
- Article author: www.physicsforums.com
- Reviews from users: 18236
Ratings
- Top rated: 4.3
- Lowest rated: 1
- Summary of article content: Articles about How could I melt Titanium? | Physics Forums Updating …
- Most searched keywords: Whether you are looking for How could I melt Titanium? | Physics Forums Updating Titanium’s melting point is between 1,600 and 1,700 degrees Celsius. What sort of equipment would I require to melt it? Is there something I can buy and…
- Table of Contents:

Titanium – Wikipedia
- Article author: en.wikipedia.org
- Reviews from users: 34518
Ratings
- Top rated: 3.9
- Lowest rated: 1
- Summary of article content: Articles about Titanium – Wikipedia Updating …
- Most searched keywords: Whether you are looking for Titanium – Wikipedia Updating
- Table of Contents:
Contents
Characteristics
Compounds
History
Production
Applications
Precautions
Function in plants
See also
References
Bibliography
External links
Navigation menu

Induction Melting of Titanium
- Article author: www.911metallurgist.com
- Reviews from users: 11504
Ratings
- Top rated: 4.2
- Lowest rated: 1
- Summary of article content: Articles about Induction Melting of Titanium Updating …
- Most searched keywords: Whether you are looking for Induction Melting of Titanium Updating
- Table of Contents:
Melting Points of Metals | OnlineMetals.com®
- Article author: www.onlinemetals.com
- Reviews from users: 32800
Ratings
- Top rated: 4.9
- Lowest rated: 1
- Summary of article content: Articles about Melting Points of Metals | OnlineMetals.com® Updating …
- Most searched keywords: Whether you are looking for Melting Points of Metals | OnlineMetals.com® Updating Learn about the importance of a melting point and the different melting points of metals including the melting point of aluminum | Online Metals®
- Table of Contents:
Common Melting Points Metals
Which Metal Has the Lowest Melting Point
Which Metal Has the Highest Melting Point
Guide to Melting Points Video
Shop the Products
Full List of All Metals and Their Melting Temperatures

titanium.org
- Article author: titanium.org
- Reviews from users: 11542
Ratings
- Top rated: 3.1
- Lowest rated: 1
- Summary of article content: Articles about titanium.org Updating …
- Most searched keywords: Whether you are looking for titanium.org Updating
- Table of Contents:

Melting Titanium Scrap | Ultraflex Power Technologies
- Article author: ultraflexpower.com
- Reviews from users: 46856
Ratings
- Top rated: 4.6
- Lowest rated: 1
- Summary of article content: Articles about Melting Titanium Scrap | Ultraflex Power Technologies Casting of titanium shavings in copper mold in order to perform an analysis of the sample. Contact us to get info about this application! …
- Most searched keywords: Whether you are looking for Melting Titanium Scrap | Ultraflex Power Technologies Casting of titanium shavings in copper mold in order to perform an analysis of the sample. Contact us to get info about this application! Casting of titanium shavings in copper mold in order to perform an analysis of the sample. Contact us to get info about this application!
- Table of Contents:

ScienceDirect
- Article author: www.sciencedirect.com
- Reviews from users: 1330
Ratings
- Top rated: 4.9
- Lowest rated: 1
- Summary of article content: Articles about ScienceDirect Currently, almost all titanium alloys are melted and cast by vacuum arc remelting (VAR) or induction skull melting using rammed graphite moulds … …
- Most searched keywords: Whether you are looking for ScienceDirect Currently, almost all titanium alloys are melted and cast by vacuum arc remelting (VAR) or induction skull melting using rammed graphite moulds …
- Table of Contents:

Induction Melting of Titanium
- Article author: www.911metallurgist.com
- Reviews from users: 16561
Ratings
- Top rated: 4.7
- Lowest rated: 1
- Summary of article content: Articles about Induction Melting of Titanium The greatest advantage of this melting technique is the capability of melting titanium sponge and scrap without fabricating an electrode. Ingots … …
- Most searched keywords: Whether you are looking for Induction Melting of Titanium The greatest advantage of this melting technique is the capability of melting titanium sponge and scrap without fabricating an electrode. Ingots …
- Table of Contents:

INDUCTION MELTING PROCESS FOR TITANIUM SCRAP (Technical Report) | OSTI.GOV
- Article author: www.osti.gov
- Reviews from users: 26298
Ratings
- Top rated: 4.8
- Lowest rated: 1
- Summary of article content: Articles about INDUCTION MELTING PROCESS FOR TITANIUM SCRAP (Technical Report) | OSTI.GOV GOV Technical Report: INDUCTION MELTING PROCESS FOR TITANIUM SCRAP … The heats were melted in a highfrequency induction furnace under an inert atmosphere. …
- Most searched keywords: Whether you are looking for INDUCTION MELTING PROCESS FOR TITANIUM SCRAP (Technical Report) | OSTI.GOV GOV Technical Report: INDUCTION MELTING PROCESS FOR TITANIUM SCRAP … The heats were melted in a highfrequency induction furnace under an inert atmosphere. The U.S. Department of Energy’s Office of Scientific and Technical Information
- Table of Contents:
Abstract
Vacuum induction melting of Ti-6Ai-4V in a cold crucible Report of Investigations1988
Induction melting and casting of titanium alloy aircraft components Final technical report Mar 1970–Jul 1972 [Ti–6Al–4V Ti–6Al–2Sn–4Zr–2Mo Ti–3Al–8V–6Cr–4Mo–4Zr (Beta C) and Ti–11Mo–4 5 Sn–6Zr (Beta 111)]
Mechanical properties of an induction-slag-melted Ti-6Al-4V alloy
EXAMINATION OF FACTORS AFFECTING THE QUALITY OF VACUUM INDUCTION-MELTED URANIUM
Consolidation of simulated nuclear metallic waste by vacuum coreless induction melting

titanium.org
- Article author: titanium.org
- Reviews from users: 32468
Ratings
- Top rated: 3.6
- Lowest rated: 1
- Summary of article content: Articles about titanium.org MELTING SYSTEMS FOR PRODUCTION OF TITANIUM INGOTS AND. CASTINGS . M. Schlienger, Retech, Inc., Ukiah, CA, USA. Titanium melting has made conserable … …
- Most searched keywords: Whether you are looking for titanium.org MELTING SYSTEMS FOR PRODUCTION OF TITANIUM INGOTS AND. CASTINGS . M. Schlienger, Retech, Inc., Ukiah, CA, USA. Titanium melting has made conserable …
- Table of Contents:

Error 403 (Forbidden)
- Article author: www.quora.com
- Reviews from users: 30123
Ratings
- Top rated: 4.6
- Lowest rated: 1
- Summary of article content: Articles about Error 403 (Forbidden) Titanium metal would not melt in any natural lavas today. Titanium melts at 1,660°C and most natural basaltic lavas are around 1,100-1,200°C. So a piece of … …
- Most searched keywords: Whether you are looking for Error 403 (Forbidden) Titanium metal would not melt in any natural lavas today. Titanium melts at 1,660°C and most natural basaltic lavas are around 1,100-1,200°C. So a piece of …
- Table of Contents:

See more articles in the same category here: 670+ tips for you.
How could I melt Titanium?
You can at minimum melt Titanium in a crucible using flux and loose cover but it is an uncertain and hazardous process .
Very spectacular when seen in a demonstration but definitely not suitable for DIY use by inexperienced people .
Titanium for serious applications is commonly melted in an induction furnace with inert gas or vacuum to prevent air contamination and actual combustion .
For critical components the entire melting and casting process is done in vacuum or inert gas environment and with controlled temperatures at all points . Cooling is also done at controlled rates .
If your proposed Titanium plate is for any engineering purpose it really needs to be a specific alloy in a specific condition .
I don’t see any actual need to cast your own – you could buy same very easily and at just a few percent of cost of trying to cast one yourself .
Wikipedia
Chemical element, symbol Ti and atomic number 22
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine.
Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of mineral deposits, principally rutile and ilmenite, which are widely distributed in the Earth’s crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils.[6] The metal is extracted from its principal mineral ores by the Kroll[7] and Hunter processes. The most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments.[8] Other compounds include titanium tetrachloride (TiCl 4 ), a component of smoke screens and catalysts; and titanium trichloride (TiCl 3 ), which is used as a catalyst in the production of polypropylene.[6]
Titanium can be alloyed with iron, aluminium, vanadium, and molybdenum, among other elements, to produce strong, lightweight alloys for aerospace (jet engines, missiles, and spacecraft), military, industrial processes (chemicals and petrochemicals, desalination plants, pulp, and paper), automotive, agriculture (farming), medical prostheses, orthopedic implants, dental and endodontic instruments and files, dental implants, sporting goods, jewelry, mobile phones, and other applications.[6]
The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element.[9] In its unalloyed condition, titanium is as strong as some steels, but less dense.[10] There are two allotropic forms[11] and five naturally occurring isotopes of this element, 46Ti through 50Ti, with 48Ti being the most abundant (73.8%).[12]
Characteristics
Physical properties
As a metal, titanium is recognized for its high strength-to-weight ratio.[11] It is a strong metal with low density that is quite ductile (especially in an oxygen-free environment),[6] lustrous, and metallic-white in color.[13] The relatively high melting point (1,668 °C or 3,034 °F) makes it useful as a refractory metal. It is paramagnetic and has fairly low electrical and thermal conductivity compared to other metals.[6] Titanium is superconducting when cooled below its critical temperature of 0.49 K.[14][15]
Commercially pure (99.2% pure) grades of titanium have ultimate tensile strength of about 434 MPa (63,000 psi), equal to that of common, low-grade steel alloys, but are less dense. Titanium is 60% denser than aluminium, but more than twice as strong[10] as the most commonly used 6061-T6 aluminium alloy. Certain titanium alloys (e.g., Beta C) achieve tensile strengths of over 1,400 MPa (200,000 psi).[16] However, titanium loses strength when heated above 430 °C (806 °F).[17]
Titanium is not as hard as some grades of heat-treated steel; it is non-magnetic and a poor conductor of heat and electricity. Machining requires precautions, because the material can gall unless sharp tools and proper cooling methods are used. Like steel structures, those made from titanium have a fatigue limit that guarantees longevity in some applications.[13]
The metal is a dimorphic allotrope of an hexagonal α form that changes into a body-centered cubic (lattice) β form at 882 °C (1,620 °F).[17] The specific heat of the α form increases dramatically as it is heated to this transition temperature but then falls and remains fairly constant for the β form regardless of temperature.[17]
Chemical properties
Like aluminium and magnesium, the surface of titanium metal and its alloys oxidize immediately upon exposure to air to form a thin non-porous passivation layer that protects the bulk metal from further oxidation or corrosion.[6] When it first forms, this protective layer is only 1–2 nm thick but it continues to grow slowly, reaching a thickness of 25 nm in four years.[19] This layer gives titanium excellent resistance to corrosion, almost equivalent to platinum.
Titanium is capable of withstanding attack by dilute sulfuric and hydrochloric acids, chloride solutions, and most organic acids.[7] However, titanium is corroded by concentrated acids.[20] As indicated by its negative redox potential, titanium is thermodynamically a very reactive metal that burns in normal atmosphere at lower temperatures than the melting point. Melting is possible only in an inert atmosphere or in a vacuum. At 550 °C (1,022 °F), it combines with chlorine.[7] It also reacts with the other halogens and absorbs hydrogen.[8]
Titanium readily reacts with oxygen at 1,200 °C (2,190 °F) in air, and at 610 °C (1,130 °F) in pure oxygen, forming titanium dioxide.[11] Titanium is one of the few elements that burns in pure nitrogen gas, reacting at 800 °C (1,470 °F) to form titanium nitride, which causes embrittlement.[21] Because of its high reactivity with oxygen, nitrogen, and many other gases, titanium that is evaporated from filaments is the basis for titanium sublimation pumps, in which titanium serves as a scavenger for these gases by chemically binding to them. Such pumps inexpensively produce extremely low pressures in ultra-high vacuum systems.
Occurrence
Titanium is the ninth-most abundant element in Earth’s crust (0.63% by mass)[22] and the seventh-most abundant metal. It is present as oxides in most igneous rocks, in sediments derived from them, in living things, and natural bodies of water.[6][7] Of the 801 types of igneous rocks analyzed by the United States Geological Survey, 784 contained titanium. Its proportion in soils is approximately 0.5 to 1.5%.[22]
Common titanium-containing minerals are anatase, brookite, ilmenite, perovskite, rutile, and titanite (sphene).[19] Akaogiite is an extremely rare mineral consisting of titanium dioxide. Of these minerals, only rutile and ilmenite have economic importance, yet even they are difficult to find in high concentrations. About 6.0 and 0.7 million tonnes of those minerals were mined in 2011, respectively.[23] Significant titanium-bearing ilmenite deposits exist in western Australia, Canada, China, India, Mozambique, New Zealand, Norway, Sierra Leone, South Africa, and Ukraine.[19] About 210,000 tonnes of titanium metal sponge were produced in 2020, mostly in China (110,000 t), Japan (50,000 t), Russia (33,000 t) and Kazakhstan (15,000 t). Total reserves of anatase, ilmenite, and rutile are estimated to exceed 2 billion tonnes.[23]
2017 production of titanium minerals and slag[23] Country thousand
tonnes % of total China 3,830 33.1 Australia 1,513 13.1 Mozambique 1,070 9.3 Canada 1,030 8.9 South Africa 743 6.4 Kenya 562 4.9 India 510 4.4 Senegal 502 4.3 Ukraine 492 4.3 World 11,563 100
The concentration of titanium is about 4 picomolar in the ocean. At 100 °C, the concentration of titanium in water is estimated to be less than 10−7 M at pH 7. The identity of titanium species in aqueous solution remains unknown because of its low solubility and the lack of sensitive spectroscopic methods, although only the 4+ oxidation state is stable in air. No evidence exists for a biological role, although rare organisms are known to accumulate high concentrations of titanium.[24]
Titanium is contained in meteorites, and it has been detected in the Sun and in M-type stars[7] (the coolest type) with a surface temperature of 3,200 °C (5,790 °F).[25] Rocks brought back from the Moon during the Apollo 17 mission are composed of 12.1% TiO 2 .[7] Native titanium (pure metallic) is very rare.[26]
Isotopes
Naturally occurring titanium is composed of five stable isotopes: 46Ti, 47Ti, 48Ti, 49Ti, and 50Ti, with 48Ti being the most abundant (73.8% natural abundance). At least 21 radioisotopes have been characterized, the most stable of which are 44Ti with a half-life of 63 years; 45Ti, 184.8 minutes; 51Ti, 5.76 minutes; and 52Ti, 1.7 minutes. All other radioactive isotopes have half-lives less than 33 seconds, with the majority less than half a second.[12]
The isotopes of titanium range in atomic weight from 39.002 u (39Ti) to 63.999 u (64Ti).[27] The primary decay mode for isotopes lighter than 46Ti is positron emission (with the exception of 44Ti which undergoes electron capture), leading to isotopes of scandium, and the primary mode for isotopes heavier than 50Ti is beta emission, leading to isotopes of vanadium.[12]
Titanium becomes radioactive upon bombardment with deuterons, emitting mainly positrons and hard gamma rays.[7]
Compounds
The +4 oxidation state dominates titanium chemistry,[28] but compounds in the +3 oxidation state are also numerous.[29] Commonly, titanium adopts an octahedral coordination geometry in its complexes,[30][31] but tetrahedral TiCl 4 is a notable exception. Because of its high oxidation state, titanium(IV) compounds exhibit a high degree of covalent bonding.[28]
Oxides, sulfides, and alkoxides
The most important oxide is TiO 2 , which exists in three important polymorphs; anatase, brookite, and rutile. All three are white diamagnetic solids, although mineral samples can appear dark (see rutile). They adopt polymeric structures in which Ti is surrounded by six oxide ligands that link to other Ti centers.[32]
The term titanates usually refers to titanium(IV) compounds, as represented by barium titanate (BaTiO 3 ). With a perovskite structure, this material exhibits piezoelectric properties and is used as a transducer in the interconversion of sound and electricity.[11] Many minerals are titanates, such as ilmenite (FeTiO 3 ). Star sapphires and rubies get their asterism (star-forming shine) from the presence of titanium dioxide impurities.[19]
A variety of reduced oxides (suboxides) of titanium are known, mainly reduced stoichiometries of titanium dioxide obtained by atmospheric plasma spraying. Ti 3 O 5 , described as a Ti(IV)-Ti(III) species, is a purple semiconductor produced by reduction of TiO 2 with hydrogen at high temperatures,[33] and is used industrially when surfaces need to be vapor-coated with titanium dioxide: it evaporates as pure TiO, whereas TiO 2 evaporates as a mixture of oxides and deposits coatings with variable refractive index.[34] Also known is Ti 2 O 3 , with the corundum structure, and TiO, with the rock salt structure, although often nonstoichiometric.
The alkoxides of titanium(IV), prepared by treating TiCl 4 with alcohols, are colorless compounds that convert to the dioxide on reaction with water. They are industrially useful for depositing solid TiO 2 via the sol-gel process. Titanium isopropoxide is used in the synthesis of chiral organic compounds via the Sharpless epoxidation.[36]
Titanium forms a variety of sulfides, but only TiS 2 has attracted significant interest. It adopts a layered structure and was used as a cathode in the development of lithium batteries. Because Ti(IV) is a “hard cation”, the sulfides of titanium are unstable and tend to hydrolyze to the oxide with release of hydrogen sulfide.[37]
Nitrides and carbides
Titanium nitride (TiN) is a refractory solid exhibiting extreme hardness, thermal/electrical conductivity, and a high melting point.[38] TiN has a hardness equivalent to sapphire and carborundum (9.0 on the Mohs scale),[39] and is often used to coat cutting tools, such as drill bits.[40] It is also used as a gold-colored decorative finish and as a barrier layer in semiconductor fabrication.[41] Titanium carbide (TiC), which is also very hard, is found in cutting tools and coatings.[42]
Halides
Titanium(III) compounds are characteristically violet, illustrated by this aqueous solution of titanium trichloride
Titanium tetrachloride (titanium(IV) chloride, TiCl 4 [43]) is a colorless volatile liquid (commercial samples are yellowish) that, in air, hydrolyzes with spectacular emission of white clouds. Via the Kroll process, TiCl 4 is used in the conversion of titanium ores to titanium metal. Titanium tetrachloride is also used to make titanium dioxide, e.g., for use in white paint.[44] It is widely used in organic chemistry as a Lewis acid, for example in the Mukaiyama aldol condensation.[45] In the van Arkel–de Boer process, titanium tetraiodide (TiI 4 ) is generated in the production of high purity titanium metal.[46]
Titanium(III) and titanium(II) also form stable chlorides. A notable example is titanium(III) chloride (TiCl 3 ), which is used as a catalyst for production of polyolefins (see Ziegler–Natta catalyst) and a reducing agent in organic chemistry.[47]
Organometallic complexes
Owing to the important role of titanium compounds as polymerization catalyst, compounds with Ti-C bonds have been intensively studied. The most common organotitanium complex is titanocene dichloride ((C 5 H 5 ) 2 TiCl 2 ). Related compounds include Tebbe’s reagent and Petasis reagent. Titanium forms carbonyl complexes, e.g. (C 5 H 5 ) 2 Ti(CO) 2 .[48]
Anticancer therapy studies
Following the success of platinum-based chemotherapy, titanium(IV) complexes were among the first non-platinum compounds to be tested for cancer treatment. The advantage of titanium compounds lies in their high efficacy and low toxicity in vivo.[49] In biological environments, hydrolysis leads to the safe and inert titanium dioxide. Despite these advantages the first candidate compounds failed clinical trials due to insufficient efficacy to toxicity ratios and formulation complications.[49] Further development resulted in the creation of potentially effective, selective, and stable titanium-based drugs.[49]
History
Titanium was discovered in 1791 by the clergyman and amateur geologist William Gregor as an inclusion of a mineral in Cornwall, Great Britain.[50] Gregor recognized the presence of a new element in ilmenite[8] when he found black sand by a stream and noticed the sand was attracted by a magnet.[50] Analyzing the sand, he determined the presence of two metal oxides: iron oxide (explaining the attraction to the magnet) and 45.25% of a white metallic oxide he could not identify.[22] Realizing that the unidentified oxide contained a metal that did not match any known element, Gregor reported his findings to the Royal Geological Society of Cornwall and in the German science journal Crell’s Annalen.[50][51][52]
Around the same time, Franz-Joseph Müller von Reichenstein produced a similar substance, but could not identify it.[8] The oxide was independently rediscovered in 1795 by Prussian chemist Martin Heinrich Klaproth in rutile from Boinik (the German name of Bajmócska), a village in Hungary (now Bojničky in Slovakia).[50][53] Klaproth found that it contained a new element and named it for the Titans of Greek mythology.[25] After hearing about Gregor’s earlier discovery, he obtained a sample of manaccanite and confirmed that it contained titanium.[54]
The currently known processes for extracting titanium from its various ores are laborious and costly; it is not possible to reduce the ore by heating with carbon (as in iron smelting) because titanium combines with the carbon to produce titanium carbide.[50] Pure metallic titanium (99.9%) was first prepared in 1910 by Matthew A. Hunter at Rensselaer Polytechnic Institute by heating TiCl 4 with sodium at 700–800 °C under great pressure[55] in a batch process known as the Hunter process.[7] Titanium metal was not used outside the laboratory until 1932 when William Justin Kroll produced it by reducing titanium tetrachloride (TiCl 4 ) with calcium.[56] Eight years later he refined this process with magnesium and with sodium in what became known as the Kroll process.[56] Although research continues to seek cheaper and more efficient routes, such as the FFC Cambridge process, the Kroll process is still predominantly used for commercial production.[7][8]
Titanium of very high purity was made in small quantities when Anton Eduard van Arkel and Jan Hendrik de Boer discovered the iodide process in 1925, by reacting with iodine and decomposing the formed vapors over a hot filament to pure metal.[57]
In the 1950s and 1960s, the Soviet Union pioneered the use of titanium in military and submarine applications[55] (Alfa class and Mike class)[58] as part of programs related to the Cold War.[59] Starting in the early 1950s, titanium came into use extensively in military aviation, particularly in high-performance jets, starting with aircraft such as the F-100 Super Sabre and Lockheed A-12 and SR-71.[60]
Throughout the Cold War period, titanium was considered a strategic material by the U.S. government, and a large stockpile of titanium sponge (a porous form of the pure metal) was maintained by the Defense National Stockpile Center, until the stockpile was dispersed in the 2000s.[61] As of 2021, the four leading producers of titanium sponge were China (52%), Japan (24%), Russia (16%) and Kazakhstan (7%).[23]
Production
Titanium (mineral concentrate)
Basic titanium products: plate, tube, rods, and powder
The processing of titanium metal occurs in four major steps: reduction of titanium ore into “sponge”, a porous form; melting of sponge, or sponge plus a master alloy to form an ingot; primary fabrication, where an ingot is converted into general mill products such as billet, bar, plate, sheet, strip, and tube; and secondary fabrication of finished shapes from mill products.[62]
Because it cannot be readily produced by reduction of titanium dioxide,[13] titanium metal is obtained by reduction of TiCl 4 with magnesium metal in the Kroll process. The complexity of this batch production in the Kroll process explains the relatively high market value of titanium,[63] despite the Kroll process being less expensive than the Hunter process.[55] To produce the TiCl 4 required by the Kroll process, the dioxide is subjected to carbothermic reduction in the presence of chlorine. In this process, the chlorine gas is passed over a red-hot mixture of rutile or ilmenite in the presence of carbon. After extensive purification by fractional distillation, the TiCl 4 is reduced with 800 °C (1,470 °F) molten magnesium in an argon atmosphere.[11] Titanium metal can be further purified by the van Arkel–de Boer process, which involves thermal decomposition of titanium tetraiodide.
2 FeTiO 3 + 7 Cl 2 + 6 C → 900 o C 2 FeCl 3 + 2 TiCl 4 + 6 CO {\displaystyle {\ce {2FeTiO3 + 7Cl2 + 6C ->[900^oC] 2FeCl3 + 2TiCl4 + 6CO}}} TiCl 4 + 2 Mg → 1100 o C Ti + 2 MgCl 2 {\displaystyle {\ce {TiCl4 + 2Mg ->[1100^oC] Ti + 2MgCl2}}}
Common titanium alloys are made by reduction. For example, cuprotitanium (rutile with copper added is reduced), ferrocarbon titanium (ilmenite reduced with coke in an electric furnace), and manganotitanium (rutile with manganese or manganese oxides) are reduced.[64]
About fifty grades of titanium alloys are designed and currently used, although only a couple of dozen are readily available commercially.[65] The ASTM International recognizes 31 grades of titanium metal and alloys, of which grades one through four are commercially pure (unalloyed). Those four vary in tensile strength as a function of oxygen content, with grade 1 being the most ductile (lowest tensile strength with an oxygen content of 0.18%), and grade 4 the least ductile (highest tensile strength with an oxygen content of 0.40%).[19] The remaining grades are alloys, each designed for specific properties of ductility, strength, hardness, electrical resistivity, creep resistance, specific corrosion resistance, and combinations thereof.[66]
In addition to the ASTM specifications, titanium alloys are also produced to meet aerospace and military specifications (SAE-AMS, MIL-T), ISO standards, and country-specific specifications, as well as proprietary end-user specifications for aerospace, military, medical, and industrial applications.[67]
Titanium powder is manufactured using a flow production process known as the Armstrong process[68] that is similar to the batch production Hunter process. A stream of titanium tetrachloride gas is added to a stream of molten sodium; the products (sodium chloride salt and titanium particles) is filtered from the extra sodium. Titanium is then separated from the salt by water washing. Both sodium and chlorine are recycled to produce and process more titanium tetrachloride.[69]
Fabrication
All welding of titanium must be done in an inert atmosphere of argon or helium to shield it from contamination with atmospheric gases (oxygen, nitrogen, and hydrogen).[17] Contamination causes a variety of conditions, such as embrittlement, which reduce the integrity of the assembly welds and lead to joint failure.[70]
Titanium is very difficult to solder directly, and hence a solderable metal or alloy such as steel is coated on titanium prior to soldering.[71] Titanium metal can be machined with the same equipment and the same processes as stainless steel.[17]
Forming and forging
Commercially pure flat product (sheet, plate) can be formed readily, but processing must take into account of the tendency of the metal to springback. This is especially true of certain high-strength alloys.[72][73] Exposure to the oxygen in air at the elevated temperatures used in forging results in formation of an brittle oxygen-rich metallic surface layer called “alpha case” that worsens the fatigue properties, so it must be removed by milling, etching, or electrochemical treatment.[74]
Applications
A titanium cylinder of “grade 2” quality
Titanium is used in steel as an alloying element (ferro-titanium) to reduce grain size and as a deoxidizer, and in stainless steel to reduce carbon content.[6] Titanium is often alloyed with aluminium (to refine grain size), vanadium, copper (to harden), iron, manganese, molybdenum, and other metals.[75] Titanium mill products (sheet, plate, bar, wire, forgings, castings) find application in industrial, aerospace, recreational, and emerging markets. Powdered titanium is used in pyrotechnics as a source of bright-burning particles.[76]
Pigments, additives, and coatings
About 95% of all titanium ore is destined for refinement into titanium dioxide (TiO
2 ), an intensely white permanent pigment used in paints, paper, toothpaste, and plastics.[23] It is also used in cement, in gemstones, as an optical opacifier in paper,[77] and a strengthening agent in graphite composite fishing rods and golf clubs.[78]
TiO
2 pigment is chemically inert, resists fading in sunlight, and is very opaque: it imparts a pure and brilliant white color to the brown or grey chemicals that form the majority of household plastics.[8] In nature, this compound is found in the minerals anatase, brookite, and rutile.[6] Paint made with titanium dioxide does well in severe temperatures and marine environments.[8] Pure titanium dioxide has a very high index of refraction and an optical dispersion higher than diamond.[7] In addition to being a very important pigment, titanium dioxide is also used in sunscreens.[13]
Aerospace and marine
Because titanium alloys have high tensile strength to density ratio,[11] high corrosion resistance,[7] fatigue resistance, high crack resistance,[79] and ability to withstand moderately high temperatures without creeping, they are used in aircraft, armor plating, naval ships, spacecraft, and missiles.[7][8] For these applications, titanium is alloyed with aluminium, zirconium, nickel,[80] vanadium, and other elements to manufacture a variety of components including critical structural parts, fire walls, landing gear, exhaust ducts (helicopters), and hydraulic systems. In fact, about two thirds of all titanium metal produced is used in aircraft engines and frames.[81] The titanium 6AL-4V alloy accounts for almost 50% of all alloys used in aircraft applications.[82]
The Lockheed A-12 and its development the SR-71 “Blackbird” were two of the first aircraft frames where titanium was used, paving the way for much wider use in modern military and commercial aircraft. A large amount of titanium mill products are used in the production of many aircraft, such as (following values are amount of raw mill products used … only a fraction of this ends up in the finished aircraft): 116 metric tons are used in the Boeing 787, 77 in the Airbus A380, 59 in the Boeing 777, 45 in the Boeing 747, 18 in the Boeing 737, 32 in the Airbus A340, 18 in the Airbus A330, and 12 in the Airbus A320.[83] In aero engine applications, titanium is used for rotors, compressor blades, hydraulic system components, and nacelles.[citation needed] An early use in jet engines was for the Orenda Iroquois in the 1950s.[better source needed][84]
Because titanium is resistant to corrosion by sea water, it is used to make propeller shafts, rigging, and heat exchangers in desalination plants;[7] heater-chillers for salt water aquariums, fishing line and leader, and divers’ knives. Titanium is used in the housings and components of ocean-deployed surveillance and monitoring devices for science and the military. The former Soviet Union developed techniques for making submarines with hulls of titanium alloys[85] forging titanium in huge vacuum tubes.[80]
Titanium is used in the walls of the Juno spacecraft’s vault to shield on-board electronics.[86]
Industrial
Welded titanium pipe and process equipment (heat exchangers, tanks, process vessels, valves) are used in the chemical and petrochemical industries primarily for corrosion resistance. Specific alloys are used in oil and gas downhole applications and nickel hydrometallurgy for their high strength (e. g.: titanium beta C alloy), corrosion resistance, or both. The pulp and paper industry uses titanium in process equipment exposed to corrosive media, such as sodium hypochlorite or wet chlorine gas (in the bleachery).[87] Other applications include ultrasonic welding, wave soldering,[88] and sputtering targets.[89]
Titanium tetrachloride (TiCl 4 ), a colorless liquid, is important as an intermediate in the process of making TiO 2 and is also used to produce the Ziegler–Natta catalyst. Titanium tetrachloride is also used to iridize glass and, because it fumes strongly in moist air, it is used to make smoke screens.[13]
Consumer and architectural
Titanium metal is used in automotive applications, particularly in automobile and motorcycle racing where low weight and high strength and rigidity are critical.[90] The metal is generally too expensive for the general consumer market, though some late model Corvettes have been manufactured with titanium exhausts,[91] and a Corvette Z06’s LT4 supercharged engine uses lightweight, solid titanium intake valves for greater strength and resistance to heat.[92]
Titanium is used in many sporting goods: tennis rackets, golf clubs, lacrosse stick shafts; cricket, hockey, lacrosse, and football helmet grills, and bicycle frames and components. Although not a mainstream material for bicycle production, titanium bikes have been used by racing teams and adventure cyclists.[93]
Titanium alloys are used in spectacle frames that are rather expensive but highly durable, long lasting, light weight, and cause no skin allergies. Many backpackers use titanium equipment, including cookware, eating utensils, lanterns, and tent stakes. Though slightly more expensive than traditional steel or aluminium alternatives, titanium products can be significantly lighter without compromising strength. Titanium horseshoes are preferred to steel by farriers because they are lighter and more durable.[94]
Titanium has occasionally been used in architecture. The 42.5 m (139 ft) Monument to Yuri Gagarin, the first man to travel in space ( ), as well as the 110 m (360 ft) Monument to the Conquerors of Space on top of the Cosmonaut Museum in Moscow are made of titanium for the metal’s attractive color and association with rocketry.[95][96] The Guggenheim Museum Bilbao and the Cerritos Millennium Library were the first buildings in Europe and North America, respectively, to be sheathed in titanium panels.[81] Titanium sheathing was used in the Frederic C. Hamilton Building in Denver, Colorado.[97]
Because of titanium’s superior strength and light weight relative to other metals (steel, stainless steel, and aluminium), and because of recent advances in metalworking techniques, its use has become more widespread in the manufacture of firearms. Primary uses include pistol frames and revolver cylinders. For the same reasons, it is used in the body of laptop computers (for example, in Apple’s PowerBook line).[98][99]
Some upmarket lightweight and corrosion-resistant tools, such as shovels, knife handles and flashlights, are made of titanium or titanium alloys.[99]
Jewelry
Relation between voltage and color for anodized titanium
Because of its durability, titanium has become more popular for designer jewelry (particularly, titanium rings).[94] Its inertness makes it a good choice for those with allergies or those who will be wearing the jewelry in environments such as swimming pools. Titanium is also alloyed with gold to produce an alloy that can be marketed as 24-karat gold because the 1% of alloyed Ti is insufficient to require a lesser mark. The resulting alloy is roughly the hardness of 14-karat gold and is more durable than pure 24-karat gold.[100]
Titanium’s durability, light weight, and dent and corrosion resistance make it useful for watch cases.[94] Some artists work with titanium to produce sculptures, decorative objects and furniture.[101]
Titanium may be anodized to vary the thickness of the surface oxide layer, causing optical interference fringes and a variety of bright colors.[102] With this coloration and chemical inertness, titanium is a popular metal for body piercing.[103]
Titanium has a minor use in dedicated non-circulating coins and medals. In 1999, Gibraltar released the world’s first titanium coin for the millennium celebration.[104] The Gold Coast Titans, an Australian rugby league team, award a medal of pure titanium to their player of the year.[105]
Medical
Because titanium is biocompatible (non-toxic and not rejected by the body), it has many medical uses, including surgical implements and implants, such as hip balls and sockets (joint replacement) and dental implants that can stay in place for up to 20 years.[50] The titanium is often alloyed with about 4% aluminium or 6% Al and 4% vanadium.[106]
Medical screws and plate used to repair wrist fractures. Scale is in centimeters.
Titanium has the inherent ability to osseointegrate, enabling use in dental implants that can last for over 30 years. This property is also useful for orthopedic implant applications.[50] These benefit from titanium’s lower modulus of elasticity (Young’s modulus) to more closely match that of the bone that such devices are intended to repair. As a result, skeletal loads are more evenly shared between bone and implant, leading to a lower incidence of bone degradation due to stress shielding and periprosthetic bone fractures, which occur at the boundaries of orthopedic implants. However, titanium alloys’ stiffness is still more than twice that of bone, so adjacent bone bears a greatly reduced load and may deteriorate.[107][108]
Because titanium is non-ferromagnetic, patients with titanium implants can be safely examined with magnetic resonance imaging (convenient for long-term implants). Preparing titanium for implantation in the body involves subjecting it to a high-temperature plasma arc which removes the surface atoms, exposing fresh titanium that is instantly oxidized.[50]
Modern advancements in additive manufacturing techniques have increased potential for titanium use in orthopedic implant applications.[109] Complex implant scaffold designs can be 3D-printed using titanium alloys, which allows for more patient-specific applications and increased implant osseointegration.[110]
Titanium is used for the surgical instruments used in image-guided surgery, as well as wheelchairs, crutches, and any other products where high strength and low weight are desirable.[111]
Titanium dioxide nanoparticles are widely used in electronics and the delivery of pharmaceuticals and cosmetics.[112]
Nuclear waste storage
Because of its corrosion resistance, containers made of titanium have been studied for the long-term storage of nuclear waste. Containers lasting more than 100,000 years are thought possible with manufacturing conditions that minimize material defects.[113] A titanium “drip shield” could also be installed over containers of other types to enhance their longevity.[114]
Precautions
Titanium is non-toxic even in large doses and does not play any natural role inside the human body.[25] An estimated quantity of 0.8 milligrams of titanium is ingested by humans each day, but most passes through without being absorbed in the tissues.[25] It does, however, sometimes bio-accumulate in tissues that contain silica. One study indicates a possible connection between titanium and yellow nail syndrome.[115]
As a powder or in the form of metal shavings, titanium metal poses a significant fire hazard and, when heated in air, an explosion hazard.[116] Water and carbon dioxide are ineffective for extinguishing a titanium fire; Class D dry powder agents must be used instead.[8]
When used in the production or handling of chlorine, titanium should not be exposed to dry chlorine gas because it may result in a titanium–chlorine fire.[117]
Titanium can catch fire when a fresh, non-oxidized surface comes in contact with liquid oxygen.[118]
Function in plants
[25] Nettles contain up to 80 parts per million of titanium.An unknown mechanism in plants may use titanium to stimulate the production of carbohydrates and encourage growth. This may explain why most plants contain about 1 part per million (ppm) of titanium, food plants have about 2 ppm, and horsetail and nettle contain up to 80 ppm.[25]
See also
References
Induction Melting of Titanium
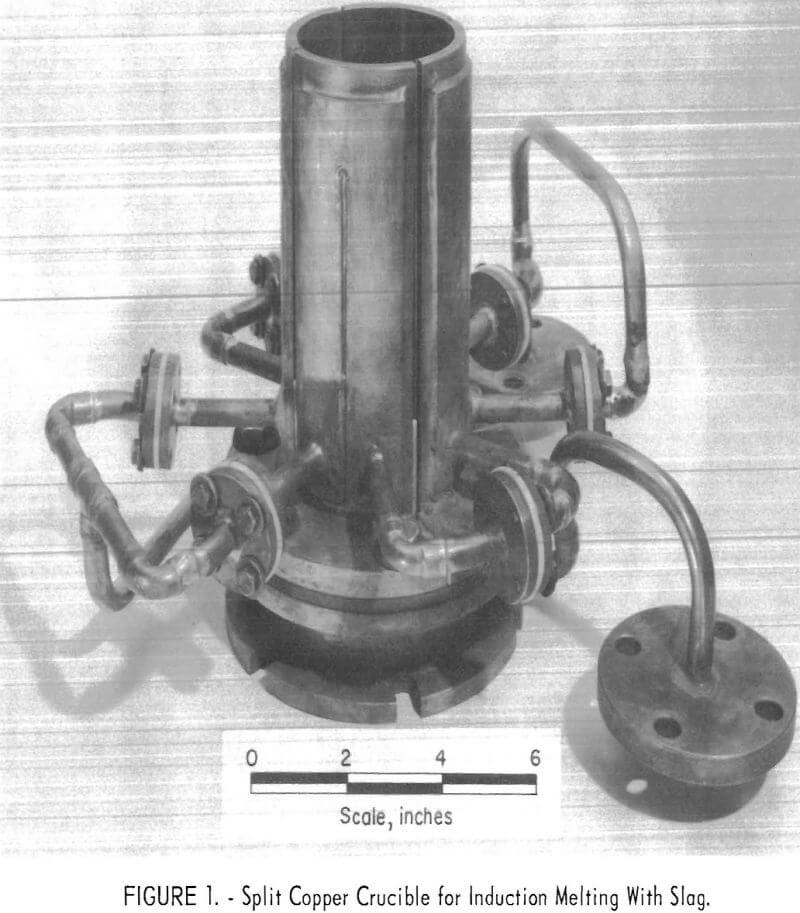
The primary purpose of the research covered by this report was the development of a method for induction melting titanium in a water-cooled copper crucible. The results reported represent findings obtained during the melting of a minimum number of ingots; therefore, only tentative conclusions can be drawn regarding the effects on metal quality of many of the operating procedures.
The most important result of the research was the development of a method for melting titanium by induction heating in a water-cooled copper crucible. The method is an improvement of the method of Schippereit, Leatherman, and Evers; a similar crucible design was used, but for this investigation a cover of slag was used to insulate the ingot from the crucible walls. The addition of this slag cover eliminated arcing between the crucible and the ingot. One run was conducted during the course of this research with no slag cover in the crucible. During this run, arcing between the ingot and the crucible segments was noted, and damage to the crucible occurred. These experimental results confirmed, the value of the slag as an electrical insulator between the ingot and the crucible.
The chief advantage of this melting technique over vacuum-arc melting and electroslag melting is the capability of melting loose titanium sponge and scrap. This capability eliminates the need for fabrication of the consumable electrode required for both vacuum-arc melting and electroslag melting. Titanium scrap can also be easily consolidated into a usable form by this technique.
One possible application of this process would be as a first-melting operation to convert titanium sponge into an electrode for vacuum-arc remelting. This would eliminate the compaction and welding steps needed for preparing the first-melt electrode and would also constitute the first-melting step in the present vacuum-arc-melting technique. Ingots prepared by this modified melting technique had slightly higher levels of impurities than ingots prepared by conventional double vacuum-arc melting. The inability of this process to remove hydrogen from sponge with high hydrogen and the residual fluorine content resulting from melting with calcium fluoride slag are considered possible serious problems to this process, but improvements in equipment and procedures should reduce other impurities to an acceptable level.
A complete study has not been made of the effect of furnace pressure on the level of impurities in titanium melted by this technique. Attempts have been made to melt at low furnace pressure with only moderate success, and most melting was done at one-third of an atmosphere of helium. Vacuum-distilled sponge melted at low pressure had lower levels of impurities than similar metal melted at a pressure of one-third of an atmosphere, but a study of the effect of intermediate pressures was not undertaken. One attempt to melt leached-and-dried sponge at low pressure was unsuccessful because of excessive outgassing of the molten pool; however, melting at intermediate pressures between zero and one-third of an atmosphere might be effective.
Results obtained with this equipment indicate that chances of satisfactory scaleup are very good; however, failure of the large-scale tests made by the Air Force based on Schippereit’s work raises a question of possible problems associated with scaleup. The authors believe that Schippereit’s small-scale tests were successful because a combination of high power input and small ingot size caused distortion of the molten metal away from the crucible walls similar to the distortion of the pool noted in this work. In the Air Force’s large-scale work; the bulk of metal melted was too large to be similarly held away from the wall, and good electrical contact between the ingot and crucible occurred. Tests conducted with and without slag in the 3-½- inch-diameter crucible confirmed both the effectiveness of the slag as an insulator and the need for the slag even on runs of this size, Barring unforeseen difficulties, the slag should be equally effective as an insulator between the ingot and the crucible in large-scale runs.
The fact that this technique makes possible the melting of loose titanium sponge and scrap is encouraging, but it is not known whether gains made by eliminating electrode fabrication would result in lower melting costs.
Further tests with this equipment are now in process; the results will be the subject of subsequent reports.
The Bureau of Mines has been active in research related to the vacuum-arc melting of reactive and refractory metals for many years and recently has actively pursued a program of research on alternate melting techniques, including techniques involving induction heating. This report describes an induction-melting technique by which titanium can be melted in a split, water-cooled copper crucible. The term “inductoslag” melting has been applied to this technique to correspond to the term “electroslag” used to describe the Hopkins process now used extensively for melting specialty steels and superalloys. As in electroslag melting, the ingot was melted under a cover of molten slag, but melting was accomplished by induction heating.
Titanium, because of its reactive nature, is melted almost exclusively by consumable electrode vacuum-arc melting. This melting process provides a relatively economical means of melting in vacuum and in water-cooled copper crucibles, but has the disadvantages of requiring the fabrication of a consumable electrode and of lack of control of the melting rate. Studies are being conducted by the Bureau to evaluate the Hopkins “electroslag” process for titanium melting.
Numerous attempts have been made to utilize induction melting for melting reactive metals. Notable among recent attempts was the work of Schippereit, Leatherman, and Evers of Battelle Memorial Institute. In their work, a segmented, water-cooled copper crucible was used to contain a charge of titanium which was heated inductively and melted. While this technique worked satisfactorily for small-scale ingots, work conducted by the U.S. Air Force on a 14-inch-diameter crucible of the same design failed because molten metal shorted across the slits in the crucible and caused crucible failure.
Earlier Bureau research resulted in the development of an induction-melting technique which did not require the use of a split crucible. Results of this work have been reported previously for melting titanium ingots up to 2 inches in diameter, and studies are still in process on melting larger titanium ingots.
The work of Schippereit probably came nearest to achieving the desired results, even though the system he proposed did not function on large-scale equipment. His work with a segmented crucible was closely related to work conducted as long ago as 1926 by Siemens, who proposed the use of a split, water-cooled crucible of this type.
The large-scale work conducted by the U.S. Air Force on Schippereit’s design also involved the unsuccessful use of an oxide coating on the interior crucible surface to prevent shorting across the crucible slits. A thin coating of beryllium oxide was placed on the inner surface of the crucible, but, when a titanium ingot was melted in the crucible, this coating failed, and the crucible was damaged by arcing between the ingot and the crucible walls. Work with this equipment was terminated because of consistent failure of the crucible whenever melting of the ingot occurred. It was concluded that arcing to the crucible walls occurred with the 14-inch-diameter crucible but not with the small-scale crucible (2-½-inch-diameter) because of the difference in frequencies used. In the small-scale studies, the frequency was 2,000 to 3,000 cycles per second (cps) as compared with only 60 cps for the large-scale work.
This report describes studies conducted in crucibles similar to those of Schippereit, however, in these studies, calcium fluoride was used as a slag cover for the ingot. Titanium ingots 3-½ inches in diameter were successfully prepared by induction melting under a cover of molten slag. A thin layer of this slag solidified against the cold wall of the crucible. This layer of solid slag provided good electrical insulation between the ingot and the crucible and eliminated all tendency for arcing between the ingot and the crucible. Invention report MIN-1296, dated November 20, 1967, describes this process; the patent application is pending.
Discussion
Description of Equipment
Based on preliminary experiments, a copper crucible with four slits was constructed, and provisions were made to enclose the unit in a vacuum chamber. This crucible, shown in figure 1, has a 3-½-inch ID, and the segmented section was 8-½ inches long. The segments of the crucible were bolted to a lower section, 2 inches long with a 3-½-inch ID. The lower section was not segmented but served as a base on which to bolt the crucible segments and to provide an additional length of cooled crucible through which the solidified ingot was withdrawn. The crucible sections were insulated from each other by an aluminum oxide thermocouple insulator, and the assembled sections were insulated from the lower furnace section by a micarta ring. The lower crucible section was bolted directly to the furnace bottom.
Figure 2 shows the crucible, complete with work coil, installed in the furnace chamber. The work coil was seven turns of ½-inch, heavy-walled
copper tubing and was insulated with Teflon tubing. With the work coil located near the top of the crucible, heating was most intense approximately 2-¼ inches below the top of the crucible. In this way, the ingot melted in the upper part of the crucible and was cooled and solidified in the lower part. Power for melting was supplied by a 10,000-cps motor-generator rated at 75 kilowatts.
The furnace body shown in figure 2 provided a controlled atmosphere for melting. A 260-cfm, single-stage blower backed by a 21-cfm mechanical pump was used to evacuate the chamber with melting normally conducted in approximately one-third of an atmosphere of helium. The furnace was also equipped with sidefeeding units for adding both calcium fluoride and titanium sponge. Ingots formed were withdrawn by the shaft through the bottom of the furnace. The top sidefeeder could be replaced with a similar shaft through the furnace top if the metal to be melted was in rod form.
Figure 3 is a longitudinal section of the crucible showing the relative position of the work coil, ingot, and slag cover during melting. As shown in the figure, melting took place at the top of the ingot. Heating of the ingot maintained a pool of molten slag at the top of the ingot although a layer of this slag solidified against the water-cooled copper wall of the crucible.
The starting stub was a 5-inch length of titanium approximately 3-¼-inch OD. This diameter, slightly less than the inside diameter of the crucible, prevented the crucible segments from being shorted out and also left space for a slag cover to form when melting started. A shoulder at the bottom of the starting stub prevented powdered or molten slag from running out of the bottom of the crucible. The starting stub was long enough for this shoulder to be below the segmented portion of the crucible.
Figure 4 is a cross section of the segmented portion of the crucible and shows the detail of the slit construction. The aluminum oxide (Al2O3) thermocouple beads shown in figure 4 were used as a convenient means of maintaining the spacing between the segments of the crucible during assembly. They also provided a seal to prevent molten slag from leaking out of the crucible. Once the molten slag ran into the slots and against these insulators, the slag solidified and formed a layer of solid calcium fluoride between the metal and the aluminum oxide.
The shape of the ingot cross section in figure 4 is representative of the portion of the ingot below the melting zone. The solidified slag around the ingot was normally 1/16 inch thick or less. During melting, the molten pool was distorted into the shape shown in figure 3. The cover of slag around the ingot was thicker near the top of the ingot, and most of the slag was molten. A thin layer of solid slag was observed at the top of the slag pool, however, and it was assumed that a continuous sleeve of solid slag existed at all times.
The levitating forces acting on the molten metal caused a part of the metal to be lifted above the surface of the molten slag. Levitation of the metal was probably exaggerated because of the buoyant forces exerted by the molten slag surrounding the metal. This levitation of the metal probably also aided in preventing metal from shorting across the slits in the crucible. The field strength was more intense at the crucible slits than in the area between two slits; consequently, metal adjacent to the slits was forced inward. When viewed from above, the metal surface protruding from the surface of the slag
had the shape of a four-pointed star with the points located midway between the slits.
The question arises as to whether the slag, once molten, was heated by induced current flow in the slag itself. The absence of any visible levitation of the slag indicated that the slag was heated only by contact with the metal.
Melting Procedure and Results
Melting experiments were conducted with a variety of forms of titanium including previously melted swaged rods, vacuum-distilled sponge, leached-and-dried sponge, and scrap. Calcium fluoride slag, prepared by fusing reagent-grade calcium fluoride, was used for all runs. A complete discussion of slag treatment is given by Ausmus and Beall.
While melting procedures varied slightly with different starting materials, the general procedure for all runs was to establish a pool of molten metal and slag in the crucible and then to add metal to this pool. Metal was added by drip melting from the lower end of a consumable feed rod or by side-feeding loose sponge metal or scrap into the top of the crucible. As material was added to the pool, the resulting ingot was withdrawn through the bottom of the crucible.
Success in melting depended on first establishing a full pool of molten metal and slag in the crucible, particularly during runs when loose sponge was added to the pool by sidefeeding. The procedure developed consisted of placing an initial charge of the metal to be melted in the crucible with the required amount of slag. The initial charge of metal was a 2-½- to 2-¾-inch-diameter cylinder approximately 3 inches long. Pressed compacts of titanium sponge were used when melting sponge, and either pressed sponge compacts or machined ingot sections were used when drip melting feed rods of previously melted titanium.
This initial charge of metal was placed on top of the 3-¼-inch-diameter starting stub previously described, and the annular space between the metal and the crucible wall was filled with granular calcium fluoride slag. The starting stub was attached to a water-cooled control rod through the bottom of the furnace, and this rod was used to adjust the level of the ingot in the crucible and to withdraw the ingot as it formed.
After the starting stub and slag were added to the crucible, the metal to be melted was loaded into the furnace either as a feed rod attached to a motor-driven control rod or as loose, granular sponge metal in a manually controlled sidefeeder. Solid feed rods were either swaged rods of previously melted material or compacted sponge bars. The furnace was evacuated and then backfilled with helium to approximately one-third of an atmosphere.
At the start of melting, the level of the initial charge of metal in the crucible was placed at the midpoint of the work coil. When this initial charge was heated inductively, the slag surrounding it melted and formed an insulating layer between the metal and the crucible wall. With sufficient heating, the metal melted and welded itself to the top surface of the starting stub. Power supplied to the work coil was limited to approximately 30 kilowatts during the first 2 or 3 minutes until the metal and slag in the crucible melted. The power level was then increased to 45 to 50 kilowatts, and, as soon as a full pool of molten metal had formed, the metal to be melted was added to the pool.
When melting from a solid rod, the rod was fed downward until its lower end was submerged in the slag in the crucible. This placed the lower end of the feed rod in the hot zone, and metal at the end melted and transferred to the pool of molten metal in the crucible. As the feed rod melted, it was fed downward, and the resulting ingot was withdrawn through the bottom of the crucible. Melting continued until the feed rod was consumed. The remaining stub of the feed rod was withdrawn from the crucible, power to the work coil was terminated, and the ingot was allowed to cool.
When the ingot was sufficiently cool, it was removed from the furnace, and the thin layer of slag which had formed on the ingot as melting progressed was removed. Figure 5 is a photograph of an ingot produced from previously melted, vacuum-distilled sponge. The feed rod for this ingot was a 2-inch- diameter swaged rod. The lower 5 inches of this ingot was the starting stub, and the upper 4-¾ inches consisted of metal drip-melted from the feed rod plus a 2-½-inch-diameter machined ingot section 2-½ inches long. Melting results for this ingot are summarized in table 1. The low melting rate and relatively high energy utilization for this ingot reflect difficulties experienced with control of the feed rod; however, better results during melting of solid feed rods could probably be obtained with additional melting experience.
When loose sponge was melted, sponge was dropped into the pool from the outlet of the sidefeeder located 11 inches above the top of the crucible. This was done as soon as a full pool of molten metal and slag had formed in the crucible. The rate of addition was limited by the rate at which the solid sponge was assimilated by the molten pool. Makeup slag was added as the slag cover was depleted by solidification of a slag layer on the outer surface of the ingot. As metal was added, the ingot was withdrawn to maintain a constant level of the ingot in the crucible, and melting was continued until the supply of sponge metal in the sidefeeder was exhausted. The ingot was allowed to cool and was then removed from the furnace.
Figure 6 shows an ingot prepared from vacuum-distilled sponge, and figure 7 shows an ingot prepared from leached-and-dried titanium sponge. The ingot melted from leached-and-dried titanium sponge was expected to be more difficult to melt than vacuum-distilled sponge because of the higher content of volatile impurities, but no serious difficulties were encountered when melting this material, although the melting rate was somewhat lower, and the ingot sidewalls were rougher. A somewhat smaller initial charge of sponge in the crucible or an initial charge of previously melted material was used to reduce excessive outgassing when melting started. Melting results for these two ingots are also summarized in table 1.
Ingots were also induction melted from titanium scrap to determine the utility of this process for reclaiming scrap. The scrap used consisted of broken tensile specimens, chopped sheet, sections of ingots, and other forms of solid titanium. No attempt was made to utilize machine chips. Figure 8 shows an ingot prepared from scrap similar to
that shown beside the ingot. Because of the difficulty of sidefeeding this scrap, the bulk of the metal melted was simply placed in the crucible along with a supply of calcium fluoride slag. The ingot shown was prepared by filling the crucible with scrap and slag three times and melting the three charges one on top of the other. No difficulties were experienced in melting this material, and the resulting ingot was excellent.
During the melting of this ingot, a sufficient number of pieces of scrap were side-fed into the molten pool to determine whether chunks of metal could be added in this manner. Ten pieces, each weighing 10 to 15 grams, were dropped into the pool from a tube approximately 11 inches above the top of the crucible. All pieces dropped quietly into the pool with virtually no splashing of metal. This was enough to show that the scrap could be added in this way if provisions were made to drop them from a shorter distance above the crucible.
Surface quality of the ingots produced by this melting technique was directly related to the quality of the starting materials, smoother surfaces being obtained from materials low in volatile impurities. The best surfaces were obtained from previously melted material, either in the form of swaged rods (fig. 5) or scrap (fig. 8). Ingots prepared from titanium sponge had slightly rougher surfaces than those prepared from melted stock, and, of the two sponge varieties studied, vacuum-distilled sponge (fig. 6) yielded ingots with better surfaces than those from leached-and-dried sponge (fig. 7).
All of the ingots were relatively sound internally except for shrink holes near the upper surface. No attempt was made to reduce the size of these shrink holes, although it is believed that hot-topping practices similar to those used for vacuum-arc melting and electroslag melting of titanium could reduce the size of these holes or possibly eliminate them entirely. Figure 9 shows the etched internal surface of an ingot produced from vacuum-distilled sponge. There was no subsurface porosity such as is characteristic of vacuum-arc-melted ingots. It would appear, from the size of the shrink hole and the large area of equiaxed grains, that the molten pool was quite large and perhaps represented almost half of the ingot shown. Further studies are needed, however, to establish accurately the boundary of the molten pool.
Sufficient data have not been obtained to determine the optimum ratio of slag to metal. This ratio varied with the type of material melted; somewhat more slag was needed for melting leached-and-dried sponge than for vacuum- distilled sponge or previously melted metal. For runs conducted in the 3-½- inch diameter crucible described, the weight of slag used was approximately 10 percent of the weight of metal melted. The ratio of slag to metal would decrease with increased ingot length since a large percentage of the slag used was tied up in the pool of slag at the top of the ingot, and the dimensions of this pool of slag would not change with ingot length. The ratio of slag to metal might also be expected to decrease with increased ingot diameter; however, no runs were conducted in which the ingot diameter was varied.
The greatest advantage of this melting technique is the capability of melting titanium sponge and scrap without fabricating an electrode. Ingots can be melted more rapidly and with fewer kilowatt-hours per pound by vacuum-arc melting and by electroslag melting but only when the titanium is in electrode form. Fabrication of consumable electrodes from titanium sponge is an expensive step in the conversion of sponge to ingot, and consolidation of titanium scrap into consumable electrodes for remelting is likewise expensive and difficult. Loose titanium sponge and scrap can be melted by this induction- melting technique with ease; in fact, it was easier to melt loose materials than solid rods.
Ingots prepared by inductoslag melting titanium sponge can be used as consumable electrodes for vacuum-arc remelting into a final ingot. Induction melting would eliminate the pressing and welding operations required to produce a first-melt electrode for vacuum-arc melting and would also constitute the initial melting step. One purpose of this work was to develop such a melting scheme and to compare the resulting ingots with ingots prepared by conventional double vacuum-arc melting.
Induction-melted ingots prepared from all the varieties of starting materials discussed previously were suitable for electrode stock for vacuum-arc remelting. No machining of the outer surface of the ingot was necessary. Ingot length was limited by the capacity of the experimental induction furnace, and it was necessary to weld several ingots together to form an electrode for vacuum-arc remelting. A typical electrode produced by welding four induction- melted ingots together is shown in figure 10. This electrode, which was approximately 3-3/8 inches in diameter and 24 inches long, was vacuum-arc remelted into the 5-inch-diameter ingot shown in figure 11. Analyses of the final ingot and of the four induction-melted ingots used for the electrode are included in table 2. Also included in table 2 are analyses of ingots prepared from the same sponge lot by conventional double vacuum-arc melting and by electroslag melting. Samples of the four induction-melted ingots were taken
from the upper portion of each ingot, and analyses of these samples are less representative of the overall ingot composition than if samples had been obtained from several locations in the ingot. Consequently, a fifth ingot, induction melt 111, was prepared from the same sponge lot by induction melting, and this ingot was more thoroughly sampled. Results of analyses of this ingot are also shown in table 2.
Analyses of a similar set of ingots prepared from leached-and-dried titanium sponge by induction melting and by other techniques are given in table 3. The titanium sponge from which these ingots were prepared was typical, magnesium-reduced domestic sponge. This sponge contains an appreciably greater quantity of hydrogen and other volatile impurities than vacuum-distilled sponge. These impurities can be reduced to acceptable levels by double or triple melting in consumable-electrode, vacuum-arc furnaces. However, ingots prepared from this sponge by electroslag melting contained up to 200 ppm hydrogen, and, for this reason, electroslag melting was not considered a satisfactory melting technique for domestic leached-and-dried sponge. Hydrogen content of the ingots produced by induction melting was only slightly lower than for ingots produced by electroslag melting.
The ingot shown in figure 12 was prepared by vacuum-arc remelting the induction-melted ingots 103 through 106. Analyses of this ingot, SA 26,512, are included in table 3. Hydrogen content of the arc-melted ingot was still above the acceptable level. The aluminum content of this ingot was appreciably higher than the corresponding ingot, SA 26,496, prepared from vacuum-distilled sponge. However, the aluminum content of the ingot (SA 26,155) prepared from this sponge by double vacuum-arc melting was also high.
Table 3 also includes analyses of a fifth induction-melted ingot, number 112, which was subjected to a more thorough analysis than the ingots used for remelting.
Most of the ingots prepared by induction melting were melted at a furnace pressure of approximately one-third of an atmosphere of helium. This furnace pressure was arbitrarily chosen to correspond with that used during studies of the electroslag melting of titanium. A partial backfill, which had been chosen for electroslag melting to reduce losses of calcium fluoride, was for the same reason chosen for induction melting. However, a number of runs were also attempted in the induction furnace at lower furnace pressures. Induction-melted ingot 108, included in table 2, was prepared from vacuum-distilled sponge at a reduced furnace pressure. For this ingot, melting was initiated at the usual one-third atmosphere pressure, but, as soon as a molten pool had been established, the vacuum valve was opened, and the furnace pressure was reduced to less than 1,000 millitorr. At this low pressure, difficulty with electrical discharges within the furnace was experienced, and the run was terminated.
A similar run was attempted with leached-and-dried titanium sponge, but outgassing of the molten pool was so violent at low pressure that no ingot was obtained. The improvement in the impurity content of ingot 108 and the obvious outgassing of the molten pool during the one run conducted at low pressure with leached-and-dried sponge indicate the need for further study of the effect of furnace pressure on metal quality. Operation at reduced furnace pressure should be the best method of lowering the hydrogen content of ingots prepared from leached-and-dried sponge. A detailed study of the effect of furnace pressure between zero and one- third atmosphere has not been undertaken at this stage of development of the melting process.
One run was also attempted in this equipment without slag in order to prove the necessity of using slag to prevent arcing between the ingot and the crucible. For this run, the usual initial charge of metal was placed in the crucible, and provisions were made to sidefeed vacuum-distilled sponge into the crucible as melting progressed. The furnace was backfilled to one-third of an atmosphere of helium, and heating of the metal in the crucible was initiated. As soon as a pool of molten metal formed and molten metal ran against the sides of the crucible, heating of the metal decreased, and it was impossible to maintain a full pool of molten metal. Sponge added to the crucible by side-feeding did not melt completely.
When the metal was removed from the crucible, evidence of arcing between adjacent segments of the crucible was noted. The slits in the crucible were pitted at the level of most intense heating, and metal which had been melted had run into the slits in the crucible. No further attempts were made to operate without slag because of the negative results obtained during this run.
So you have finished reading the how to melt titanium topic article, if you find this article useful, please share it. Thank you very much. See more: how to melt titanium in terraria, titanium forging, titanium melting temp, how to make titanium, titanium investment casting, titanium breaking, titanium die casting, titanium axe

