Contents
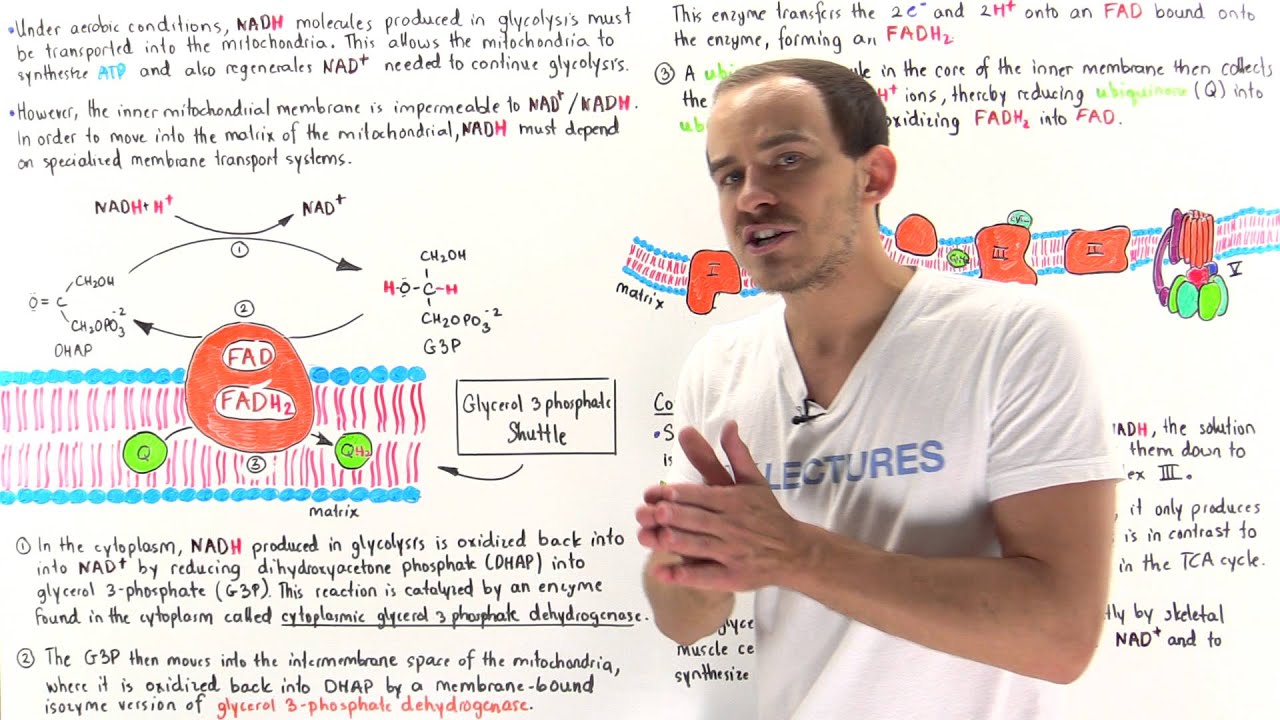
Glycerol 3-Phosphate Shuttle
นอกจากการดูบทความนี้แล้ว คุณยังสามารถดูข้อมูลที่เป็นประโยชน์อื่นๆ อีกมากมายที่เราให้ไว้ที่นี่: ดูความรู้เพิ่มเติมที่นี่
Donate here: http://www.aklectures.com/donate.php
Website video: http://www.aklectures.com/lecture/glycerol3phosphateshuttle
Facebook link: https://www.facebook.com/aklectures
Website link: http://www.aklectures.com
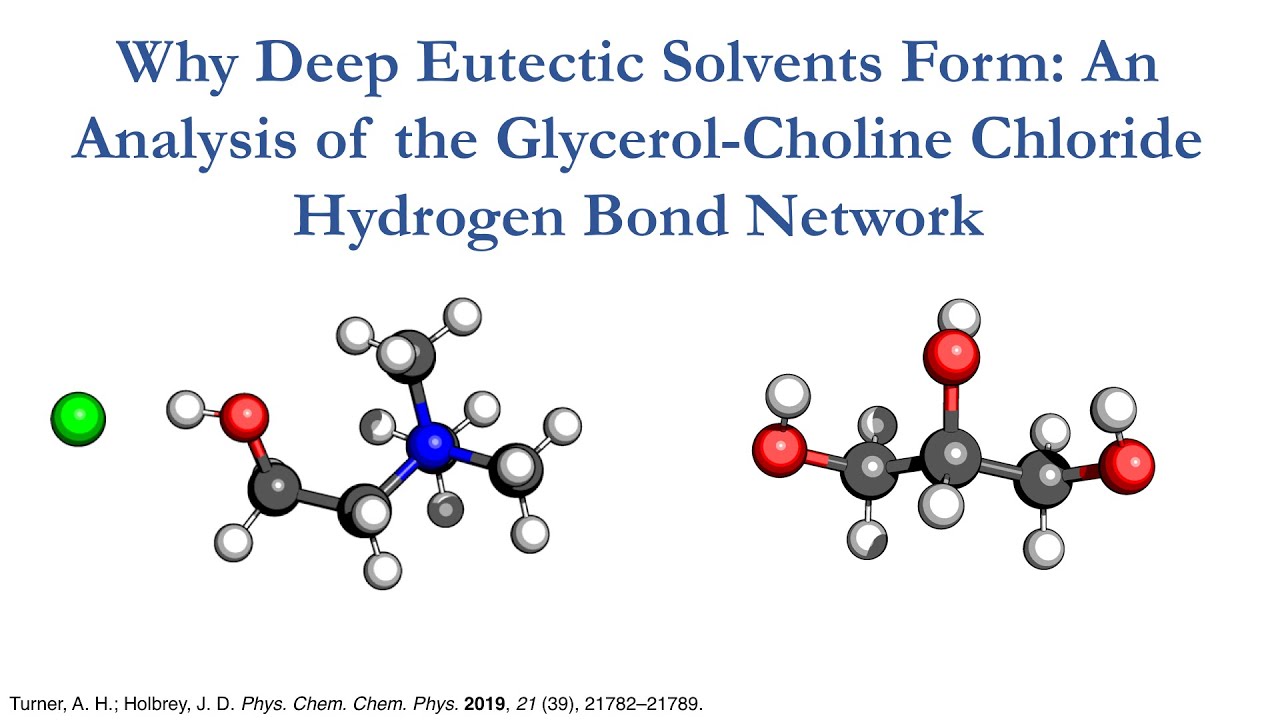
Why Deep Eutectic Solvents Form: An Analysis of the Glycerol-Choline Chloride Hydrogen Bond Network
Originally prepared for VCTC 2020, this is my lightning talk on a recent paper done with John Holbrey on the choline chloride glycerol hydrogenbond network using small angle neutron diffraction.
Abstract:
\”The structure of choline chloride/glycerol (ChCl : Gly) mixtures at two mole fractions (the eutectic χChCl = 0.33 (1 : 2), and a higher χChCl = 0.50 (1 : 1) composition) in the liquid state at 333 K and 1 atm. has been investigated using neutron diffraction coupled with hydrogen/deuterium isotopic substitution. Modelling using the empirical potential structure refinement (EPSR) technique, constrained to the experimental neutron diffraction data, produced structural models at both compositions consistent with the experimental data with an extensive, persistent homomolecular glycerol hydrogen bonding network at χChCl = 0.33 similar to that present in pure glycerol and suggests that persistence of the latent glycerol hydrogen bonding network is key to formation of the ChCl : Gly deep eutectic solvent. In the choline chloriderich χChCl = 0.50 composition, significant domain segregation is observed with a dramatic reduction in the extent of the homomolecular glycerol hydrogen bond network which is replaced by a more homogeneous systemwide hydrogen bonded network incorporating glycerol, Cl−, and choline cations.\”
The original paper can be found at: https://pubs.rsc.org/en/content/articlelanding/2019/cp/c9cp04343h/
Saponification: The process of Making Soap – MeitY OLabs
This video channel is developed by Amrita University’s CREATE
http://www.amrita.edu/create
▶ For more Information @
http://amrita.olabs.edu.in/?sub=73\u0026brch=3\u0026sim=119\u0026cnt=1
▶ Amrita Online Lab Project Website
http://www.olabs.edu.in/
▶ Subscribe @
https://www.youtube.com/user/amritacreate
▶ Like us @
https://www.facebook.com/CREATEatAmrita
Copyright © 2017 Amrita University
Developed by Amrita University \u0026 CDAC Mumbai. Funded by MeitY (Ministry of Electronics \u0026 Information Technology)
Saponification: The process of Making Soap :
The term saponification is the name given to the chemical reaction that occurs when a vegetable oil or animal fat is mixed with a strong alkali. The products of the reaction are two: soap and glycerin. The name saponification literally means \”soap making\”. Different oils are used in saponification process. Coconut oil creates lots of glycerin, makes big bubbly lather, and is very stable. Olive oil has natural antioxidants and its soap makes a creamier lather. The alkali used in soap is either potassium hydroxide, which is used to make soft soap or liquid soap because of its greater solubility, or sodium hydroxide, which is used to make hard soap.
This video explains the preparation of soap by saponification reaction.

Очистка глицерина сырца – Purification of raw glycerol
Детали и консультация (Details and consultation:) https://litechaqua.com/ochistka_ghlitsierina
telegram/whatsapp/viber: +38093 661 31 21

How to make Glycerine (Glycerol)
This video was requested by a commenter…I don’t remember who, but this video is for you! I went with the method of producing biodiesel, but you could also go with simple saponification (different procedure though).
The process is very slow and long. It is much easier to simply buy glycerol from the store. It would be much faster and much more pure than making it yourself.
Glycerine can be made from triglycerides in vegetable oils. Most biofats are stored as triglycerides which are made of 3 fatty acids and a glycerol backbone. This is the same method as making biodiesel from vegetable oil. where glycerine is normally considered a byproduct.
Nile talks about lab safety: https://youtu.be/ftACSEJ6DZA
• NileRed Store: https://bit.ly/3BHx06i
• Patreon: https://www.patreon.com/nilered
• Youtube Membership: https://bit.ly/3zQX7Xm
• NileRed Newsletter: https://nile.red/homenewsletter
• Discord: https://discord.gg/3BT6UHf
• Facebook: https://www.facebook.com/NileRed2
• Instagram: https://www.instagram.com/nile.red
• Snapchat: https://bit.ly/3ieuf5e
• TikTok: https://www.tiktok.com/@nilered
• Twitter: https://twitter.com/NileRed2

นอกจากการดูหัวข้อนี้แล้ว คุณยังสามารถเข้าถึงบทวิจารณ์ดีๆ อื่นๆ อีกมากมายได้ที่นี่: ดูวิธีอื่นๆTIPS

